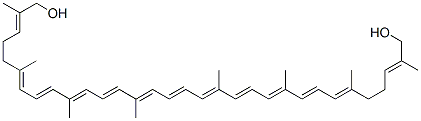
lycophyll synthesis
- Product Name:lycophyll
- CAS Number:19891-75-9
- Molecular formula:C40H56O2
- Molecular Weight:568.87
40772-87-0
0 suppliers
inquiry

19891-75-9
1 suppliers
inquiry
Yield:19891-75-9 38%
Reaction Conditions:
Stage #1: dimethyl Ψ,Ψ-carotene-16,16'-dioatewith diisobutylaluminium hydride in tetrahydrofuran;toluene at 0 - 20; for 1 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran;toluene; for 0.5 h;
Steps:
15
Example 15 Preparation of ψ,ψ-Carotene-16,16'-diol (2H) To a solution of dimethyl ester 218a (1.14 g, 1.83 mmol) in anhydrous tetrahydrofuran (100 mL) at 0° C. was added DIBAL (20% by wt. in toluene) (9.13 μL, 11.0 mmol) via syringe. The mixture was warmed to room temperature and stirred for one hour. The reaction was quenched by the sequential addition of H2O (440 μL), 15% aqueous NaOH (440 μL), and H2O (1.10 mL). The resulting mixture was stirred for 30 minutes and then dried over anhydrous MgSO4. After filtration and removal of solvent in vacuo, the resulting crude diol 2H (0.39 g, 38%) was used in the next step without further purification. 1H NMR (500 MHz, CDCl3) δ: 6.63 (d of d, J=15.0 Hz, J=11.5 Hz, 2H, H11, H11'), 6.63 (d, J=11.0 Hz, 2H, H15, H15'), 6.48 (d of d, J=15.0 Hz, J=11.0 Hz, 2H, H7, H7'), 6.36 (d, J=15.0 Hz, 2H, H12, H12'), 6.25 (d, J=15.0 Hz, 2H, H8, H8'), 6.19 (d, J=11.5 Hz, 2H, H10, H10'), 5.95 (d, J=11.0 Hz, 2H, H6, H6'), 5.40 (t of q, J=6.50 Hz, J=1.50 Hz, 2H, H2, H2'), 4.00 (s, 4H, -CH2O-), 2.19 (t, J=Hz, 4H, -CH2-), 2.16 (t, J=Hz, 4H, -CH2-), LC/MS (APCI): m/z 569 [M+H]+.
References:
US2008/221377,2008,A1 Location in patent:Page/Page column 40; 20
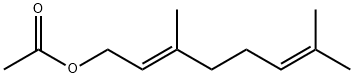
105-87-3
417 suppliers
$8.00/25g

19891-75-9
1 suppliers
inquiry
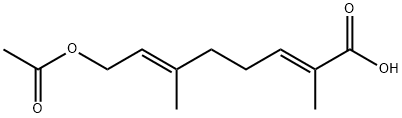
83852-61-3
0 suppliers
inquiry

19891-75-9
1 suppliers
inquiry

26187-80-4
31 suppliers
inquiry

19891-75-9
1 suppliers
inquiry

40772-83-6
0 suppliers
inquiry

19891-75-9
1 suppliers
inquiry