
M-AMINOBENZYL CYANIDE synthesis
- Product Name:M-AMINOBENZYL CYANIDE
- CAS Number:4623-24-9
- Molecular formula:C8H8N2
- Molecular Weight:132.16
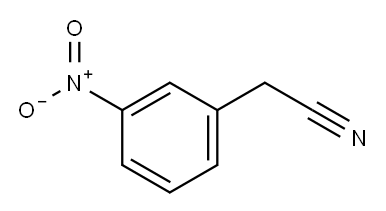
621-50-1
150 suppliers
$10.00/1g

4623-24-9
74 suppliers
$51.32/0.25g
Yield: 98%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 85; for 1 h;
Steps:
General Procedure for the Preparation of 3,4-dialkylaniline; 3-Methyl-4-(2-(methylthio)ethoxy)aniline (10a), Method A.
General procedure: To a solution of 2 (150.1 mg, 0.66 mmol) and NH4Cl (183.6 mg, 3.43 mmol) in a mixture of EtOH/H2O (5:1, 6 mL) was added iron (Fe) powder (184.4 mg, 3.30 mmol) and vigorously stirred at 85 °C for 1 h. After the mixture was allowed to cool to room temperature, the solid was removed by filtration through a Celite pad, and the filtrate was concentrated. The residue was purified by column chromatography (EtOAc/hexane, 20:70) to afford 10a (99.2 mg, 76%) as a brown oil;
References:
Suebsuwong, Chalada;Pinkas, Daniel M.;Ray, Soumya S.;Bufton, Joshua C.;Dai, Bing;Bullock, Alex N.;Degterev, Alexei;Cuny, Gregory D. [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 4,p. 577 - 583] Location in patent:supporting information
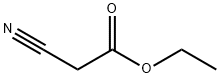
105-56-6
432 suppliers
$10.00/5ml
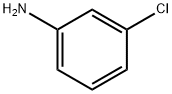
108-42-9
449 suppliers
$10.00/5g

4623-24-9
74 suppliers
$51.32/0.25g
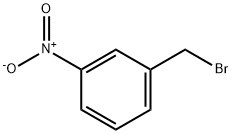
3958-57-4
238 suppliers
$17.00/10g

4623-24-9
74 suppliers
$51.32/0.25g

5247-25-6
4 suppliers
inquiry

4623-24-9
74 suppliers
$51.32/0.25g
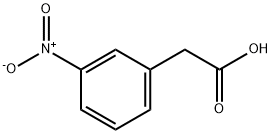
1877-73-2
197 suppliers
$24.00/1g

4623-24-9
74 suppliers
$51.32/0.25g