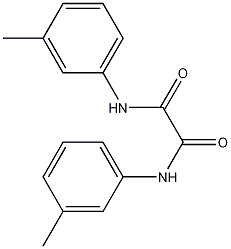
m-Oxalotoluidide synthesis
- Product Name:m-Oxalotoluidide
- CAS Number:3551-75-5
- Molecular formula:C16H16N2O2
- Molecular Weight:268.31

79-37-8
461 suppliers
$17.67/10gm:
![4-[(3-methylphenyl)amino]-4-oxobutanoic acid](/CAS/GIF/62134-48-9.gif)
62134-48-9
14 suppliers
$45.00/5mg
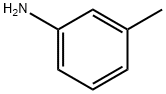
108-44-1
369 suppliers
$9.00/1g

3551-75-5
8 suppliers
$505.63/5MG
Yield: 23%
Reaction Conditions:
Stage #1:oxalyl dichloride;N-m-tolylsuccinamic acid in 1,4-dioxane at 0 - 20; for 2.5 h;
Stage #2:1-amino-3-methylbenzene with pyridine in 1,4-dioxane at 0 - 20; for 24.5 h;
Stage #3: with water;sodium hydrogencarbonate in 1,4-dioxaneSaturated solution;
Steps:
4.2.3. Preparation of N,N-di-oxalamide derivatives
General procedure: To a magnetically stirred ice-bath cooled solution of the selected succinic monoamide derivative (4 mmol) in anhydrous dioxane (20 ml) neat oxalyl chloride was added (8 mmol). After stirring over 30 min the reaction mixture was warmed to room temperature. Two hours later, the reaction mixture was re-cooled to 0 °C in ice bath followed by drop wise addition of the particular aromatic amine (4 mmol) in pyridine (10 mL) to the reaction mixture. After stirring over 30 min the reaction mixture was warmed to room temperature and allowed to stir over 24 h. Subsequently, the reaction was carefully quenched with saturated sodium bicarbonate solution (200 mL). Finally, the mixture was filtered to offer the products as whitish to gray solids that were re-crystallized from acetone (see Scheme 2).
References:
Habash, Maha;Taha, Mutasem O. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 16,p. 4746 - 4771] Location in patent:experimental part

79-37-8
461 suppliers
$17.67/10gm:

108-44-1
369 suppliers
$9.00/1g

3551-75-5
8 suppliers
$505.63/5MG
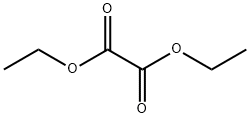
95-92-1
408 suppliers
$5.00/5 g

108-44-1
369 suppliers
$9.00/1g

17738-79-3
10 suppliers
inquiry

3551-75-5
8 suppliers
$505.63/5MG
