
m-Phenylenediamine synthesis
- Product Name:m-Phenylenediamine
- CAS Number:108-45-2
- Molecular formula:C6H8N2
- Molecular Weight:108.14
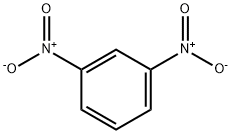
99-65-0
4 suppliers
$32.00/25g

108-45-2
443 suppliers
$13.00/25g
Yield:108-45-2 100%
Reaction Conditions:
with hydrogen in ethanol at 20; under 760.051 Torr; for 3 h;chemoselective reaction;
Steps:
Catalytic Experiment
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.
References:
Redina;Vikanova [Russian Journal of Physical Chemistry,2018,vol. 92,# 12,p. 2374 - 2378][Zh. Fiz. Khim.,2018,vol. 92,# 12,p. 1846 - 1850,5]
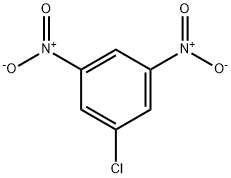
618-86-0
20 suppliers
inquiry

108-45-2
443 suppliers
$13.00/25g
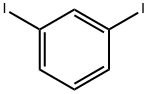
626-00-6
234 suppliers
$10.00/1g

108-45-2
443 suppliers
$13.00/25g
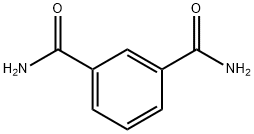
1740-57-4
71 suppliers
$21.75/1g

108-45-2
443 suppliers
$13.00/25g
97-00-7
12 suppliers
$17.67/10gm:

108-45-2
443 suppliers
$13.00/25g