
m-Tolunitrile synthesis
- Product Name:m-Tolunitrile
- CAS Number:620-22-4
- Molecular formula:C8H7N
- Molecular Weight:117.15

620-23-5
325 suppliers
$5.00/5g

620-22-4
225 suppliers
$14.00/25mL
Yield:620-22-4 99.5%
Reaction Conditions:
with oxygen;ammonium bicarbonate in dimethyl sulfoxide at 120; for 8 h;Sealed tube;Green chemistry;
Steps:
2.3. Activity test
General procedure: The typical experimental procedure for the ammoxidation of aldehydeswith ammonium carbonate was as follows: powdered catalyst (10mg), aldehydes (0.2 mmol), ammonium salt (0.4 mmol), and 2 mL solventwere added into a glass tube (10 mL) under an O2 atmosphere. Themixture in this sealed glass tube was reacted at a suitable temperaturefor determined reaction times. Then, the reaction mixture in the sealedtube was centrifuged to obtain the liquid phase, which was analyzed onan Agilent 7890B gas chromatograph (GC) that was equipped with aflame ionization detector and an HP-5 capillary column (30 m, 0.32 mminner diameter, and 0.25 μm film), and using methylbenzene as an internalstandard. The temperature program of the column oven was asfollows: from an initial temperature of 50 C, the oven was heated to180 C at a rate of 15 Cmin 1 and subsequently heated to 280 C at arate of 20 Cmin 1. The conversion and product selectivity werecalculated as follows. The pure product was isolated by silica-gel column chromatographyusing petroleum ether and ethyl acetate as eluents. The obtained purecompounds were characterized by proton nuclear magnetic resonance(1H NMR) spectroscopy on a BURCKER AVANCE II 500 M spectrometerat 20 C using CDCl3 as the solvent.
References:
Fu, Wenqian;Pan, Liuming;Tang, Tiandi;Wang, Siming;Zhang, Lei [Molecular catalysis,2022,vol. 518,art. no. 112087]
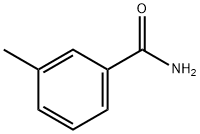
618-47-3
83 suppliers
$25.00/5g

620-22-4
225 suppliers
$14.00/25mL
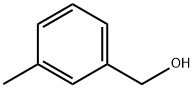
587-03-1
198 suppliers
$21.00/5g

620-22-4
225 suppliers
$14.00/25mL
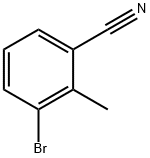
52780-15-1
132 suppliers
inquiry

620-22-4
225 suppliers
$14.00/25mL
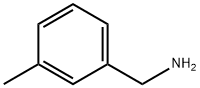
100-81-2
195 suppliers
$6.00/1g

620-22-4
225 suppliers
$14.00/25mL