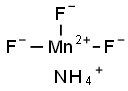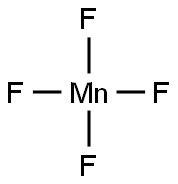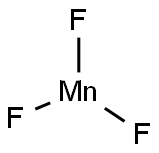
MANGANESE(III) FLUORIDE synthesis
- Product Name:MANGANESE(III) FLUORIDE
- CAS Number:7783-53-1
- Molecular formula:F3Mn
- Molecular Weight:111.93
1313-13-9
485 suppliers
$13.00/5g
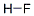
7664-39-3
449 suppliers
$17.30/25ml

7783-53-1
85 suppliers
$22.39/5G
Yield:-
Reaction Conditions:
in hydrogen fluoridedissolving freshly pptd. MnO2 in aq. HF-soln.;;
References:
Christensen, O. T. [Journal fur praktische Chemie (Leipzig. 1834),1887,vol. 35,p. 57 - 82]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7783-53-1
85 suppliers
$22.39/5G
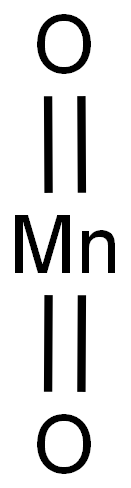
1313-13-9
485 suppliers
$13.00/5g
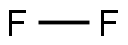
7782-41-4
0 suppliers
inquiry

7783-53-1
85 suppliers
$22.39/5G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry