
Marbofloxacin EP Impurity B synthesis
- Product Name:Marbofloxacin EP Impurity B
- CAS Number:115551-41-2
- Molecular formula:C12H8F2N2O4
- Molecular Weight:282.2
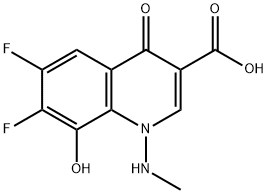
115551-40-1
28 suppliers
inquiry

115551-41-2
27 suppliers
inquiry
Yield:-
Reaction Conditions:
with paraformaldehyde in 1,4-dioxane;N-methyl-acetamide;
Steps:
1 Preparation of 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[3,2,1-ij]-1,3,4-benzoxadiazine-6-carboxylic acid
EXAMPLE 1 Preparation of 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[3,2,1-ij]-1,3,4-benzoxadiazine-6-carboxylic acid A mixture of 6,7-difluoro-8-hydroxy-1-(methylamino)-4-oxo-1,4-dihydro -3-quinolinecarboxylic acid (105 mg) obtained in Reference example (i). paraformaldehyde (150 mg) and dry dioxane (5 ml) was heated at 100° C. for 3 hours under nitrogen atmosphere. After removal of the solvent under reduced pressure dimethylformamide (20 ml) was added to the residue and the mixture was stirred for 20 minutes and then filtered. The filtered cake was washed with dimethylformamide and the combined filtrate was evaporated under reduced pressure. The residue was triturated with water and filtered to give 97 mg of 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro -7H-pyrido[3,2,1-ij]-1,3,4-benzoxadiazine-6-carboxylic acid. An analytical sample, mp 290°-292° C. (dec.); MS m/z 282 (M+) was prepared by recrystallization from dimethylformamide.
References:
US4801584,1989,A