
MESO-1,2-BIS(4-BROMOPHENYL)ETHANEDIAMINE synthesis
- Product Name:MESO-1,2-BIS(4-BROMOPHENYL)ETHANEDIAMINE
- CAS Number:117903-53-4
- Molecular formula:C14H14Br2N2
- Molecular Weight:370.08
![Benzamide, N-[1,2-bis(4-bromophenyl)-2-[[(4-bromophenyl)methylene]amino]ethyl]-4-bromo-](/CAS/20210111/GIF/364342-32-5.gif)
364342-32-5
0 suppliers
inquiry

117903-53-4
12 suppliers
inquiry
Yield:117903-53-4 66%
Reaction Conditions:
Stage #1: 4-bromo-N-(2-(4-bromobenzylideneamino)-1,2-(4-bromophenyl)ethyl)benzamidewith sulfuric acid in water; for 3 h;Microwave irradiation;Sealed tube;Heating;
Stage #2: with sodium hydroxide in water at 20; for 2 h;
Steps:
2.4
Compound 3 (2.5 g, 3.47 mmol) was taken in 3 mL of sulfuric acid and 10 mL of water in a sealed tube and heated with microwaves at 80 °C for 3 h.
The residue was cooled and precipitate was filtered off.
The crude precipitate was dissolved in 10 (M) sodium hydroxide solutions and stirred for further 2 h at room temperature.
After that the residue was dissolved in dichloromethane (150 mL) and washed with water (3 * 150 mL).
The organic phase was dried, evaporated to dryness and purified on a silica gel column using 3% isopropanol in ethyl acetate as eluent.
The mixture of epimers 4, 0.85 g was obtained in 66% yield. 1H NMR 4 (CDCl3) δ: 7.46 (4H, d, J = 8.1 Hz, ArH), 7.20 (4H, d, J = 8.1 Hz, ArH), 3.98 (4H, s, NH2), 1.38 (2H, s, CH). MS: (M+1) calculated 371.08, found 371.10.
References:
Ghosh, Pradip;Zhang, Jiawei;Shi, Zheng-Zheng;Li, King [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 8,p. 2418 - 2425]
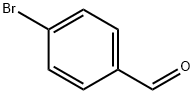
1122-91-4
670 suppliers
$9.00/10g

117903-53-4
12 suppliers
inquiry