
MESO-1,2-BIS(P-TOLYL)ETHYLENEDIAMINE synthesis
- Product Name:MESO-1,2-BIS(P-TOLYL)ETHYLENEDIAMINE
- CAS Number:50764-59-5
- Molecular formula:C16H20N2
- Molecular Weight:240.34
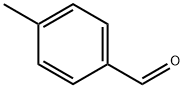
104-87-0
520 suppliers
$6.00/25g

50764-59-5
9 suppliers
inquiry
Yield:50764-59-5 70%
Reaction Conditions:
Stage #1: 4-methyl-benzaldehydewith ammonium acetate at 120; for 3 h;
Stage #2: with sulfuric acid;water at 170; for 12 h;
Steps:
5 General procedure for the chemical synthesis of diamines 2a-h
General procedure: This is an adapted protocol for previous research carried out bydifferent authors.8 A suspension of a previously distilled benzaldehyde1a-h (50 mmol) and ammonium acetate (150 mmol) washeated at 120 C, and stirred for 3 h. After this time, the reactionwas cooled to room temperature, and the gummy residue waswashed with hexane. The resulting crude was basified with anaqueous 4 M NaOH solution (pH >10) and extracted with Et2O(4 20 mL). The organic phases were combined, dried, and filtered,and the solvent was evaporated under reduced pressure.Without further purification, the resulting intermediate was suspendedin an aqueous 50% H2SO4 solution (40 mL), and the mixturewas heated overnight at 170 C. The reaction was then cooleddown in an ice-bath with stirring, and H2O (20 mL) was slowlyadded. The resulting solution was warmed until room temperatureand extracted with Et2O (4 60 mL). The aqueous phase was thenneutralized with a concentrated aqueous ammonia solution, andthen extracted with Et2O (4 60 mL). The organic phases werecombined, dried, and filtered, and the solvent was evaporated underreduced pressure, to give the corresponding meso-diamine 2a-h as a white, yellow, or brown solid (40-98% isolated yield,Table 1).4.2.5
meso-1,2-Bis(4-methylphenyl)-1,2-ethanediamine 2e
White solid (2.11 g, 70% isolated yield). Rf (60% MeOH/EtOAc): 0.47; mp: 76-78 °C; IR (KBr): νmax/cm-1 3370, 2920, 1606, 1266, 1020, 890, 735; δH (300.13 MHz, CDCl3, Me4Si): 2.01 (br s, 4H), 2.76 (s, 6H), 4.38 (s, 2H), 7.57 (d, 3JHH = 7.9 Hz, 4H), 7.69 (d, 3JHH = 7.9 Hz, 4H); δC (75.5 MHz, CDCl3, Me4Si): 21.4 (2CH3), 62.0 (2CH), 127.8 (4CH), 129.4 (4CH), 137.5 (2C), 140.3 (2C); MS (ESI+, m/z): 241.1 [(M+H)+, 100%]; HRMS (ESI+, m/z) calcd for C16H21N2 (M+H)+: 241.1699 found: 241.1687.
References:
Mendez-Sanchez, Daniel;Rios-Lombardia, Nicolas;Garcia-Granda, Santiago;Montejo-Bernardo, Jose;Fernandez-Gonzalez, Alfonso;Gotor, Vicente;Gotor-Fernandez, Vicente [Tetrahedron Asymmetry,2014,vol. 25,# 4,p. 381 - 386]
![PHENOL, 2,2''-[[1,2-BIS(4-METHYLPHENYL)1,2-ETHANEDIYL]BIS(NITRILOMETHYLIDYNE)]BIS-R,S](/CAS/GIF/58520-37-9.gif)
58520-37-9
2 suppliers
inquiry

50764-59-5
9 suppliers
inquiry