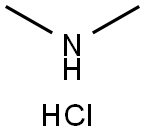
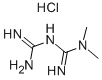
Metformin synthesis
- Product Name:Metformin
- CAS Number:657-24-9
- Molecular formula:C4H11N5
- Molecular Weight:129.16
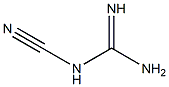
1115-70-4
813 suppliers
$5.00/5g

657-24-9
167 suppliers
$100.00/50mg
Yield:657-24-9 100%
Reaction Conditions:
with sodium hydroxide in water at 20; for 0.5 h;
Steps:
VI Example VI Preparation of metformin-L-glutamic acid-R (+) lipoic acid salt
Example VI Preparation of metformin-L-glutamic acid-R (+) lipoic acid salt Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride, 5.25 g, 0.032 mol) was stirred in 1N sodium hydroxide (32 mL, 0.032 mol) at room temperature for 30 min. Water was removed from the mixture under vacuo. The crude mixture was treated with ethanol (100 mL) and stirred for 10 min. The white residue was filtered off and ethanol was removed under vacuum to get a white residue. The ethanol treatment was repeated again to get a white solid (quantitative), which was dried in pump and used as metformin free base. Method 1: Metformin free base (1.29 g, 0.010 mol) was taken in methanol (15 mL) and while stirring L-Glutamic acid (0.74 g, 0.050 mol) was added as solid. Lipoic acid (1.03 g, 0.050 mol) was separately dissolved in methanol (15 mL) and added to this mixture through a in-line syringe filter (to remove polymeric material, if any). The mixture was continued to stir for 30 min. Leaving the mixture in the refrigerator did not cause any precipitation of the salt, hence the solvent was removed under vacuo and dried in high vacuum pump. Metformin.L-Glutamic acid.R-Lipoic acid salt (2.9 g) was obtained as light yellow foamy solid. This solid is high hygroscopic and turns into gummy residue on exposure to air.Preparation of metformin-L-glutamic acid-R (+) lipoic acid saltVI200This solid is high hygroscopic and turns into gummy residue on exposure to air. The residue was suspended in acetonitrile (30 mL) and stirred for 18 h. Filtered the pale yellow solid and dried in vacuum for 16 h to get 2.32 g of 3. 1H NMR (200 MHz, D2O) - 1.15-2.1 (10H, m), - 2.1-2.42 (5H, m), -2.88 (12H, s), - 2.95-3.12 (18, m) - 3.48-3.62 (2H, m); 13C NMR (200 MHz, D2O) -c 25.86, 27.25.28.68, 33.81, 34.14, 37.66, 38.34, 40.51, 54.95, 56.93, 158.63, 160.28, 174.80, 181.44, 183.81. Method 2: Lipoic acid (1.03 g, 0.050 mol) was dissolved in acetonitrile (30 mL) and to this Metformin free base (1.29 g, 0.010 mol) and L-Glutamic acid (0.74 g, 0.050 mol) were added as solid.Preparation of metformin-L-glutamic acid-R (+) lipoic acid saltVI200Method 2: Lipoic acid (1.03 g, 0.050 mol) was dissolved in acetonitrile (30 mL) and to this Metformin free base (1.29 g, 0.010 mol) and L-Glutamic acid (0.74 g, 0.050 mol) were added as solid. The resulting mixture was stirred for 2 h and filtered. The pale yellow solid was dried in vacuo for 16 h to get 2.56 g of 3. 1H NMR (200 MHz, D2O) - 1.15-2.1 (10H, m), - 2.1-2.42 (5H, m), - 2.88 (12H, s), - 2.95-3.12 (1H, m) - 3.48-3.62 (2H, m); 13C NMR (200 MHz, D2O) -c 25.86, 27.25, 28.68, 33.81, 34.14, 37.66, 38.34, 40.51, 54.95, 56.93, 158.63, 160.28, 174.80, 181.44, 183.81.
References:
US2014/221467,2014,A1 Location in patent:Page/Page column 4-5
![Guanidine, N-[1-(dimethylamino)ethenyl]-, hydrochloride (1:1)](/CAS/20211123/GIF/1378856-94-0.gif)
1378856-94-0
0 suppliers
inquiry

657-24-9
167 suppliers
$100.00/50mg
![Cyclohexanesulfenamide, N-[[[(dimethylamino)iminomethyl]amino]iminomethyl]-](/CAS/20210305/GIF/1170667-82-9.gif)
1170667-82-9
0 suppliers
inquiry

657-24-9
167 suppliers
$100.00/50mg
![Benzenesulfenamide, N-[[[(dimethylamino)iminomethyl]amino]iminomethyl]-](/CAS/20210305/GIF/1170667-83-0.gif)
1170667-83-0
0 suppliers
inquiry

657-24-9
167 suppliers
$100.00/50mg