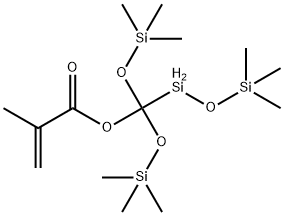
METHACRYLOXYMETHYLTRIS(TRIMETHYLSILOXY)SILANE synthesis
- Product Name:METHACRYLOXYMETHYLTRIS(TRIMETHYLSILOXY)SILANE
- CAS Number:74681-63-3
- Molecular formula:C14H34O5Si4
- Molecular Weight:394.76

107-46-0
457 suppliers
$10.00/10g
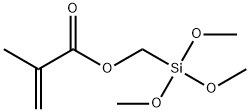
54586-78-6
59 suppliers
$499.87/5MG

74681-63-3
34 suppliers
inquiry
Yield:74681-63-3 78%
Reaction Conditions:
Stage #1: Hexamethyldisiloxane;(trimethoxysilyl)methyl-2-methylacrylatewith acetic acid;trifluorormethanesulfonic acid at 45; for 2 h;
Stage #2: with acetyl chloride at 40; for 0.333333 h;
Stage #3: with 1,1,1,3,3,3-hexamethyl-disilazane
Steps:
2
EXAMPLE 21-methacryloxymethyltris(trimethylsiloxy)silaneIn a three-necked flask, 80.8 g (0.499 mol) of hexamethyldisiloxane, 39.9 g (0.67 mol) of acetic acid and 0.5 g of a 10% strength solution of trifluoromethane-sulfonic acid in acetic acid (0.0003 mol) are initially introduced and heated to 45° C. with stirring. Then, over the course of one hour (at 45° C.), 48.8 g (0.22 mol) of 1-methacryloxymethyltrimethoxysilane (WACKER GENIOSIL XL 33) are added and after-stirred for 1 h at 45° C. The crude product is analyzed by means of gas chromatography (GC). Besides the readily volatile constituents of the reaction mixture, the following GC contents are found: product (44.7 area %); monoorganoxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OMe, 1.1 area %); monohydroxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OH, 0.7 area %) and 0.6 area % of the difunctional disiloxane (dimers) [CH2CH(CH3)-COO-(CH2)3-Si(OSiMe3)2]O. At 40° C., 6.1 g (0.078 mol) of acetyl chloride are added to the reaction mixture and then the mixture is after-stirred for 20 min. At room temperature, an acidic lower phase of the organic layer is separated off before the reaction mixture is analyzed again by gas chromatography. The following are found: product (49.3 area %); monoorganoxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OMe, 0.2 area %); monohydroxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OH, 0.7 area %) and 0.9 area % of the difunctional disiloxane (dimers) [CH2CH(CH3)-COO-CH2-Si(OSiMe3)2]O.In order to eliminate/reduce the remaining content of monohydroxysilane impurity, 17.9 g (0.11 mol) of hexamethyldisilazane are added, the reaction mixture being neutralized at the same time. After filtering off the formed salt, the reaction mixture is analyzed again by means of GC. The following are found (besides readily volatile constituents): product 48.7 area %; monoorganoxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OMe, 0.2 area %); monohydroxy impurity (CH2CH(CH3)-COO-CH2-Si(OSiMe3)2OH, 0.0 area %) and 0.9 area % of the difunctional disiloxane (dimers) [CH2CH(CH3)-COO-CH2-Si(OSiMe3)2]O. After distilling off the volatile constituents, the product is distilled in vacuo and filtered off over a filter candle (pore width 0.2 μm). The desired product is obtained in a yield of 78% and has a purity of 99.3 area % (also comprises 0.2 area % monoorganoxysilane). The monohydroxy impurity can no longer be detected).
References:
US2010/41909,2010,A1 Location in patent:Page/Page column 6