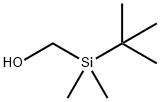
Methanol, [(1,1-diMethylethyl)diMethylsilyl]- synthesis
- Product Name:Methanol, [(1,1-diMethylethyl)diMethylsilyl]-
- CAS Number:144314-37-4
- Molecular formula:C7H18OSi
- Molecular Weight:146.3
123061-64-3
37 suppliers
inquiry
![Methanol, [(1,1-diMethylethyl)diMethylsilyl]-](/CAS/20150408/GIF/144314-37-4.gif)
144314-37-4
13 suppliers
inquiry
Yield:144314-37-4 57%
Reaction Conditions:
with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran;hexane at 0; for 0.05 h;Inert atmosphere;
Steps:
5.1 Step 1: [tert-Butyl(dimethyl)silyl]methanol
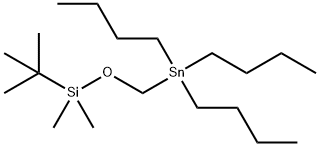
In a heat gun-dried round -bottom flask under nitrogen, -butyl lithium (1 mL, 1.600 mmol, 1.6 M solution in hexanes) was combined with N,N,N',N'- tetramethylethane-l, 2-diamine (200 pL, 1.325 mmol) in anhydrous THF (8 mL) at 0 °C in an ice-water bath. ie/ -Butyl-dimethyl-(tributylstannylmethoxy)silane (500 mg, 1.149 mmol) was then added dropwise by syringe over 1 minute. The reaction mixture was stirred for 2 minutes at 0 °C then quenched with acetic acid (200 pL, 3.517 mmol). The reaction mixture was then diluted with a saturated aqueous solution of sodium bicarbonate (10 mL), water (10 mL) and ethyl acetate (15 mL) and warmed to room temperature. The layers were separated and the aqueous phase was extracted an additional 2 x 15 mL ethyl acetate. The combined organics were washed with brine, dried over sodium sulfate, and concentrated to give a colorless oil. This crude material was purified by chromatography on silica gel (1-70% ethyl acetate in hexanes gradient, with an initial hexane flush) to give as a white solid, [/
References:
WO2019/195739,2019,A1 Location in patent:Paragraph 00205

123061-64-3
37 suppliers
inquiry

100-52-7
953 suppliers
$20.37/250g
![Methanol, [(1,1-diMethylethyl)diMethylsilyl]-](/CAS/20150408/GIF/144314-37-4.gif)
144314-37-4
13 suppliers
inquiry