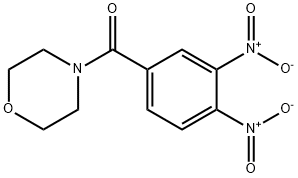
Methanone, (3,4-dinitrophenyl)-4-Morpholinyl- synthesis
- Product Name:Methanone, (3,4-dinitrophenyl)-4-Morpholinyl-
- CAS Number:65003-28-3
- Molecular formula:C11H11N3O6
- Molecular Weight:281.22
Yield:65003-28-3 100%
Reaction Conditions:
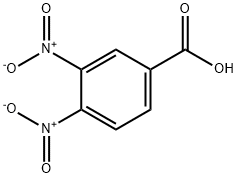
Stage #1: 3,4-dinitrobenzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane;
Stage #2: morpholine in tetrahydrofuran at 0 - 20; for 3.16667 h;Inert atmosphere;
Steps:
A A. (3, 4-dinitrophenyl)(morpholino)methanone
A. (3, 4-dinitrophenyl)(morpholino)methanone To a suspension of 3,4-dinitrobenzoic acid (1.30 g, 6.1 mmol) in anh DCM (50 mL) at rt was added dropwise (COCl)2 (1.0 mL, 11.7 mmol) followed by anh DMF (2 drops). The reaction was stirred ovemig then concentrated at rt. The residue was dissolved in anh THF (24 mL) at 0 °C under Ar. morpholine (1.0 mL, 11.6 mmol) was added dropwise (thick white suspension was stirred with intermittent shaking). After the addition the cooling was continued for 10 min before the cooling bath was removed. After stirring the reaction at rt for 3 h, H20 was added. THF was removed under reduced pressure and the aqueous residue was extracted (CH2C12. 2x). The combined organic extracts were dried (Na2S04) and concentrated under reduced pressure to afford (3,4-dinitrophenyl)(morpholino)methanone as a light orange solid (1.8 g, quant) . NMR (400 MHz, DMSO-c e) δ 8.28 - 8.31 (m, 2 H), 8.00 (dd, J=8.28, 1.76 Hz, 1 H), 3.39 - 3.80 (m, 8 H).
References:
WO2016/205942,2016,A1 Location in patent:Page/Page column 37

110-91-8
687 suppliers
$9.00/1g

65003-28-3
9 suppliers
inquiry
528-45-0
162 suppliers
$10.00/1g

65003-28-3
9 suppliers
inquiry
