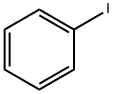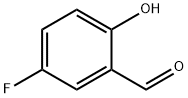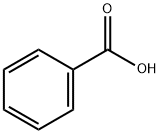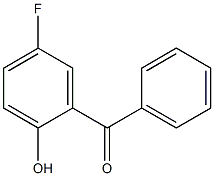
Methanone, (5-fluoro-2-hydroxyphenyl)phenyl- synthesis
- Product Name:Methanone, (5-fluoro-2-hydroxyphenyl)phenyl-
- CAS Number:362-47-0
- Molecular formula:C13H9FO2
- Molecular Weight:216.2078
Yield:362-47-0 64.2%
Reaction Conditions:
with sodium hydrogencarbonate;lithium chloride;palladium dichloride in N,N-dimethyl-formamide at 110;Inert atmosphere;
Steps:
(3,4-difluorophenyl)(2-hydroxy-4-methoxyphenyl)methanone
General procedure: A vial was charged with 2-Hydroxy-4-methoxybenzaldehyde (1.97 mmol, 300 mg), PdCl2 (5 mol%, 17.5 mg), 1,2-Difluoro-4-iodobenzene (2 equiv., 946.7 mg), Na2CO3 (2 equiv., 418.1 mg), LiCl (0.4 equiv., 16.7 mg), and DMF (19.7 mL, 0.1 M of the aldehyde), purged with N2 and stirred at 110 °C 4-10 h. The reaction was monitored with LC-MS and TLC (TLC conditions: Aliquot was diluted with CH3OH, eluted with EtOAc/heptane 1:3, and stained with 2,4- dinitrophenylhydrazine solution). The reaction mixture was filtered over a pad of Celite, diluted with EtOAc, washed 3 times with water, and the aqueous layers was acidified and extracted twice with EtOAc. The combined organic layers was dried over Na2SO4, concentrated and purified on silica using EtOAc/Heptane 1:20 → 1:9 step gradient) to afford 2'-hydroxybenzophenone in 69.3% yield. (NMR data is given in the supporting information).
References:
Saleeb, Michael;Mojica, Sergio;Eriksson, Anna U.;Andersson, C. David;Gylfe, ?sa;Elofsson, Mikael [European Journal of Medicinal Chemistry,2018,vol. 143,p. 1077 - 1089] Location in patent:supporting information
METHANONE](/CAS/GIF/10425-05-5.gif)
10425-05-5
18 suppliers
$277.00/1g

362-47-0
6 suppliers
inquiry
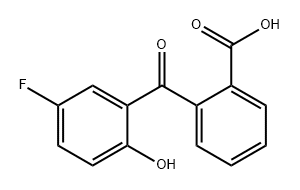
78343-73-4
0 suppliers
inquiry

362-47-0
6 suppliers
inquiry
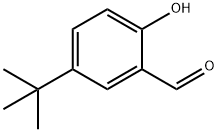
2725-53-3
142 suppliers
$20.00/1g

362-47-0
6 suppliers
inquiry