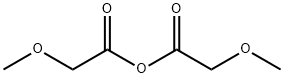
methoxyacetic anhydride synthesis
- Product Name:methoxyacetic anhydride
- CAS Number:19500-95-9
- Molecular formula:C6H10O5
- Molecular Weight:162.14
625-45-6
322 suppliers
$16.00/25g

19500-95-9
87 suppliers
$28.00/5G
Yield: 34%
Reaction Conditions:
with acetic anhydride at 100; for 2 h;
Steps:
1
6,7-Dimethoxy-1-methoxymethyl-isoquinolin-3-ol SMA 44012 A mixture of methoxyacetic acid (21.9 g, 242.67 mmol) and acetic anhydride (24.80 g, 242.67 mmol) in a 250 mL round-bottomed flask equipped with a magnetic stirrer and a distillation assembly was heated with stirring for 2 h at 100° C. and then distilled under reduced pressure. Methoxyacetic anhydride (13.5 g, 34% yield) was collected at 108° C. at around 20 mb. To a solution of methyl 2-(3,4-dimethoxy-phenyl)acetate SLA 28134 (3.36 g, 15.98 mmol) in methoxyacetic anhydride SMA 44010 (10.19 g, 62.85 mmol) at 0° C. in a 250 mL round-bottomed flask equipped with a magnetic stirrer was added dropwise HClO4 (ca. 70% solution in water, 1.64 mL, 18.98 mmol). The mixture was then stirred for 45 min at RT then diluted with Et2O (151 mL). The organic solution was removed to give a viscous residue that was suspended in H2O (30 mL) at 5° C. before dropwise addition of a conc. NH4OH solution (38 mL). After complete addition, the mixture was stirred overnight at RT and extracted with CH2Cl2 (50 mL) then with CH2Cl2:MeOH=95:5 (100 mL). The organic phase was combined, washed with brine (30 mL), dried over Na2SO4, filtered and concentrated at 40° C. under vacuum. Purification by column chromatography (SiO2, eluent CH2Cl2:MeOH=100:0 to 93:7) gave 6,7-dimethoxy-1-methoxymethyl-isoquinolin-3-ol SMA 44012 as a yellow solid (96 mg, 2% yield). MW: 249.27; Yield: 2%; Yellow Solid. Mp (° C.): 214.1 (dec.) Rf (free base)=0.2 (CH2Cl2:MeOH=95:5). 1H NMR (CDCl3:CD3OD=2:1, δ): 3.57 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.88 (s, 2H, CH2), 6.65 (s, 1H, ArH), 6.74 (s, 1H, ArH), 6.87 (s, 1H, ArH). 13C-NMR (CDCl3:CD3OD=2:1, δ): 57.4, 57.6, 60.5, 70.3, 102.3, 104.2, 107.9, 114.5, 143.9, 145.2, 150.0, 156.9, 161.8. MS-ESI m/z (% rel. Int.): 250 ([MH]+, 100).
References:
ExonHit Therapeutics SA;ALLERGAN, INC. US2012/214837, 2012, A1 Location in patent:Page/Page column 83

108-24-7
0 suppliers
$14.00/250ML

625-45-6
322 suppliers
$16.00/25g

19500-95-9
87 suppliers
$28.00/5G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

19500-95-9
87 suppliers
$28.00/5G

108-24-7
0 suppliers
$14.00/250ML
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

625-45-6
322 suppliers
$16.00/25g

19500-95-9
87 suppliers
$28.00/5G

64-19-7
1628 suppliers
$10.00/25ML