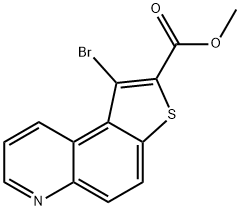
Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate synthesis
- Product Name:Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate
- CAS Number:1188365-72-1
- Molecular formula:C13H8BrNO2S
- Molecular Weight:322.18
![Thieno[3,2-f]quinoline-2-carboxylic acid, 1-amino-, methyl ester](/CAS/20200611/GIF/1188365-71-0.gif)
1188365-71-0
2 suppliers
inquiry
![Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate](/CAS/20180703/GIF/1188365-72-1.gif)
1188365-72-1
15 suppliers
inquiry
Yield:1188365-72-1 40%
Reaction Conditions:
Stage #1: methyl 1-aminothieno[3,2-f]quinoline-2-carboxylatewith tert.-butylnitrite in acetonitrile at 0; for 1 h;
Stage #2: with copper(ll) bromide in acetonitrile at 0 - 20; for 3 h;
Steps:
S1-Step 4 S1-Step 4: Synthesis of Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate (S1-5)
To a solution of methyl 1-aminothieno[3,2-f]quinoline-2-carboxylate (S1-4) (40 g, 154.8 mmol) in acetonitrile (1000 mL), tert-butyl nitrite (27.6 mL, 232 mmol) was added dropwise at 0° C. and stirred for 1 h at 0° C. To the resulting mixture, copper (II) bromide (41.5 g, 185.8 mmol) was added portionwise at 0° C. and stirred at room temperature for 3 h. After completion, the reaction mixture was diluted with water (3.0 L) and extracted with 2% methanol in chloroform (3.0 L). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford the title compound S1-5 (20 g, 40%) as a pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 3.93 (s, 3H), 7.77 (dd, J=4.2 Hz, 8.7 Hz, 1H), 8.14 (d, J=9.0 Hz, 1H), 8.40 (d, J=9.0 Hz, 1H), 9.02 (d, J=3.1 Hz, 1H), 10.10 (d, J=8.8 Hz, 1H). MS m/z (M+H): 323.9
References:
US2016/75720,2016,A1 Location in patent:Paragraph 0229; 0233
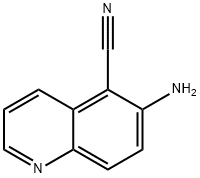
54398-51-5
30 suppliers
$30.50/250mg
![Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate](/CAS/20180703/GIF/1188365-72-1.gif)
1188365-72-1
15 suppliers
inquiry
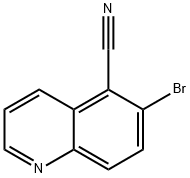
1188365-70-9
21 suppliers
$52.50/250mg
![Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate](/CAS/20180703/GIF/1188365-72-1.gif)
1188365-72-1
15 suppliers
inquiry
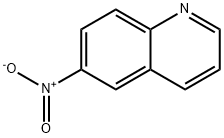
613-50-3
238 suppliers
$10.00/5g
![Methyl 1-bromothieno[3,2-f]quinoline-2-carboxylate](/CAS/20180703/GIF/1188365-72-1.gif)
1188365-72-1
15 suppliers
inquiry