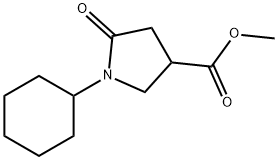
Methyl 1-cyclohexyl-5-oxopyrrolidine-3-carboxylate synthesis
- Product Name:Methyl 1-cyclohexyl-5-oxopyrrolidine-3-carboxylate
- CAS Number:100252-83-3
- Molecular formula:C12H19NO3
- Molecular Weight:225.28
Yield:100252-83-3 82%
Reaction Conditions:
in methanol; for 16 h;Heating / reflux;
Steps:
14.A
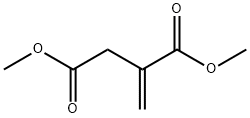
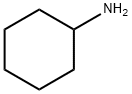
A mixture of cyclohexylamine (12 g), dimethyl itaconate (21.1 g) and methanol (80 mL) was heated under reflux for 16 hr. After cooling, the reaction mixture was concentrated under reduced pressure, diluted hydrochloric acid was added to the residue, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography (hexane-ethyl acetate 2:3-1:4) to give the title compound (22.3 g, 82%) as a pale-yellow oil. 1H NMR (300 MHz, CDCl3)δppm 1.05-1.15 (m, 1H), 1.29-1.46 (m, 4H), 1.64-1.72 (m, 3H), 1.78-1.82 (m, 2H), 2.60-2.76 (m, 2H), 3.14-3.25 (m, 1H), 3.49- 3.59 (m, 2H), 3.74 (s, 3H), 3.89-4.00 (m, 1H).
References:
EP1864971,2007,A1 Location in patent:Page/Page column 126