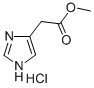
METHYL 2-(1H-IMIDAZOL-4-YL)ACETATE HYDROCHLORIDE synthesis
- Product Name:METHYL 2-(1H-IMIDAZOL-4-YL)ACETATE HYDROCHLORIDE
- CAS Number:51718-80-0
- Molecular formula:C6H9ClN2O2
- Molecular Weight:176.6

67-56-1
738 suppliers
$9.00/25ml
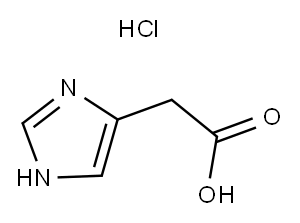
3251-69-2
206 suppliers
$15.00/250mg

51718-80-0
23 suppliers
$275.00/250mg
Yield:51718-80-0 100%
Reaction Conditions:
with hydrogenchloride at 20; for 5.5 h;
Steps:
5
4-lmidazoleacetic acid hydrochloride (5.93 g, 36.47 mmol) in methanol (125 mL) was saturated with HCI gas and stirred at room temperature for 5.5 h, then concentrated to give a quantitative yield of 4-imidazoleacetic acid methyl ester hydrochloride as a white solid which had NMR (MeOH- d4) δ 8.87 (d, J = 1.2 Hz, 1H), 7.46 (s, 1 H), 3.89 (s, 2H) 3.72
References:
WO2007/34277,2007,A1 Location in patent:Page/Page column 56

67-56-1
738 suppliers
$9.00/25ml
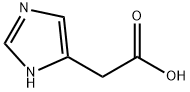
645-65-8
87 suppliers
inquiry

51718-80-0
23 suppliers
$275.00/250mg

3251-69-2
206 suppliers
$15.00/250mg

51718-80-0
23 suppliers
$275.00/250mg

645-65-8
87 suppliers
inquiry

51718-80-0
23 suppliers
$275.00/250mg