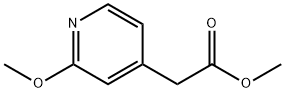
Methyl 2-(2-methoxypyridin-4-yl)acetate synthesis
- Product Name:Methyl 2-(2-methoxypyridin-4-yl)acetate
- CAS Number:464152-37-2
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
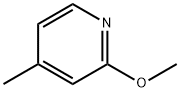
100848-70-2
169 suppliers
$10.00/1g
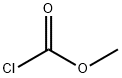
79-22-1
2 suppliers
$38.00/1000g

464152-37-2
29 suppliers
inquiry
Yield:464152-37-2 44%
Reaction Conditions:
Stage #1: 2-methoxy-4-methyl-pyridinewith lithium diisopropyl amide in tetrahydrofuran at -60; for 2 h;Inert atmosphere;
Stage #2: methyl chloroformate in tetrahydrofuran at 20; for 1 h;
Steps:
16.1 step 1 2-(2-methoxypyridin-4-yl) methyl acetate 16b
2-methoxy-4-methylpyridine 16a (2.0g, 16.2mmol)Dissolved in anhydrous tetrahydrofuran (20mL),Under the protection of nitrogen, the temperature is reduced to -60,Slowly add lithium diisopropylamide (20.4mL, 40.8mmol, 2M tetrahydrofuran solution),Stir at -60°C for 2 hours,Add methyl chloroformate (2.0g, 21.2mmol),After the addition is complete, stir at room temperature for 1 hour.TLC showed that the reaction was complete.The reaction solution was poured into water, extracted with ethyl acetate, and the organic phases were combined,Wash with saturated brine, dry with anhydrous sodium sulfate, concentrate,The crude silica gel column chromatography gave the title compound 16b (1.3 g, yield 44%) as a yellow liquid.
References:
CN113173924,2021,A Location in patent:Paragraph 0480-0487