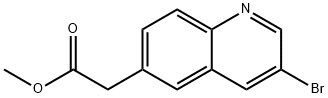
Methyl 2-(3-bromoquinolin-6-yl)acetate synthesis
- Product Name:Methyl 2-(3-bromoquinolin-6-yl)acetate
- CAS Number:1022091-89-9
- Molecular formula:C12H10BrNO2
- Molecular Weight:280.12
5622-36-6
77 suppliers
$22.00/100mg

1022091-89-9
22 suppliers
$378.00/100mg
Yield:1022091-89-9 45.3%
Reaction Conditions:
Stage #1: methyl 2-(quinolin-6-yl)acetatewith bromine in tetrachloromethane; for 4 h;Heating / reflux;
Stage #2: with pyridine in tetrachloromethane; for 2 h;Product distribution / selectivity;Heating / reflux;
Steps:
2
To a stirred solution of quinolin-6-yl-acetic acid methyl ester (21.6 g, 107 mmol) in 150 ml of carbon tetrachloride was treated with bromine (34.4 g, 215 mmol) and heated to reflux for 4 hours. The reaction mixture was treated with 17.0 g of pyridine, and further stirred for 2 hours under reflux. After cooling down to ambient temperature, the mixture was partitioned between dichloromethane and saturated aqueous sodium hydrogen carbonate, the organic layer was washed with water and brine, dried over magnesium sulfate then evaporated under reduced pressure to give a brown residue. The residue was purified by column chromatography, eluting with petroleum (60~90°C) and then a 30:1 mixed solvent of petroleum and ethyl acetate to return title compound (13.6 g, 45.3%) as a white crystalline solid. (300MHz, DMSO-d6): 8.89 (d, IH), 8.27 (d, IH), 8.06 (d, IH), 7.67~7.64(m, 2H), 3.82(s, 2H), 3.72(s, 3H). ES-MS m/z: 280 (M+H+).
References:
WO2008/144767,2008,A1 Location in patent:Page/Page column 115

67-56-1
737 suppliers
$9.00/25ml

1022091-93-5
44 suppliers
$45.00/10mg

1022091-89-9
22 suppliers
$378.00/100mg