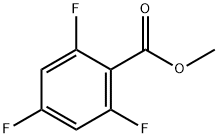
Methyl 2,4,6-trifluorobenzoate synthesis
- Product Name:Methyl 2,4,6-trifluorobenzoate
- CAS Number:79538-28-6
- Molecular formula:C8H5F3O2
- Molecular Weight:190.12

67-56-1
737 suppliers
$9.00/25ml
28314-80-9
308 suppliers
$6.00/1g

79538-28-6
31 suppliers
$13.00/250mg
Yield:79538-28-6 85%
Reaction Conditions:
with thionyl chloride at 65; for 72 h;Inert atmosphere;Cooling with ice;
Steps:
4.2.3. General procedure for esterifications
General procedure: A solution of 2,4,6-trifluorobenzoic acid (47) or 2,3,4-trifluorobenzoic acid (48) (0.3 g, 1.7 mmol) in dry methanol (10 mL) was prepared in a three-necked, round-bottom flask fitted with a reflux condenser and a calcium chloride tube. The flask was placed in an ice/water bath, thionyl chloride (1.4 mL) was then added dropwise, and the mixture was heated at 60-65 °C for three days under argon. After removal of solvent, the resulting residue was purified by extraction with pentane in the case of 49 and using column chromatography on silica gel (hexane/diethyl ether 6:1) for 50.Methyl 2,4,6-trifluorobenzoate (49): Colorless oil, yield 85% (lit. [41] Bp 105-110 °C/15 mmHg). 1H NMR (CDCl3) δ 3.94 (s, 3H, OCH3), 6.72 (dd, 3J = 3J = 8.3, 2H, H3 and H5).
References:
Claramunt, Rosa M.;López, Concepción;López, Ana;Pérez-Medina, Carlos;Pérez-Torralba, Marta;Alkorta, Ibon;Elguero, José;Escames, Germaine;Acu?a-Castroviejo, Darío [European Journal of Medicinal Chemistry,2011,vol. 46,# 4,p. 1439 - 1447] Location in patent:experimental part