
Methyl 2-(4-cyano-3-fluorophenyl)acetate synthesis
- Product Name:Methyl 2-(4-cyano-3-fluorophenyl)acetate
- CAS Number:444807-50-5
- Molecular formula:C10H8FNO2
- Molecular Weight:193.17
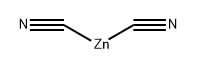
557-21-1
106 suppliers
$12.00/5g
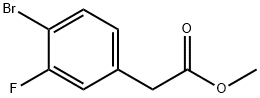
942282-41-9
41 suppliers
$163.00/250mg

444807-50-5
13 suppliers
inquiry
Yield:444807-50-5 60.2%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 155; for 1.5 h;Inert atmosphere;Microwave irradiation;
Steps:
35.2 Step 2: methyl 4-cyano-3-fluorophenylacetate
Ethyl-4-bromo-3-fluorophenylacetate (1.3 g, 5.3 mmol), zinc cyanide (924 mg, 7.89 mmol) and N,N-dimethylformamide (10 mL) were added to a 20 mL microwave tube. The mixture was nitrogen sparged for 5 min, tetrakis(triphenylphosphine)palladium (613 mg, 0.53 mmol) was added, and the mixture was stirred and heated to 155 °C for 1.5 hours under microwave. After completion of reaction, the mixture was cooled to room temperature, and extracted with ethyl acetate for three times, and the organic layers were combined, washed 5 times with water, and finally washed with saturated sodium chloride, and the organic layer was concentrated in vacuo to get a crude product. Then the crude product was separated by a silica gel column (petroleum ether: ethyl acetate = 8:1-5:1) to give the product (while solid, 620 mg), with a yield of 60.2%. 1H NMR(400 MHz, CDCl3) 7.58 (t, J= 7.2 Hz, 1H), 7.18 (d, J= 8.7 Hz, 2H), 3.72 (s, 3H), 3.69 (s, 2H). MS (ESI) m/z: 194.1(MH+)
References:
EP3476829,2019,A1 Location in patent:Paragraph 0216

942282-41-9
41 suppliers
$163.00/250mg

557-21-1
106 suppliers
$12.00/5g

444807-50-5
13 suppliers
inquiry
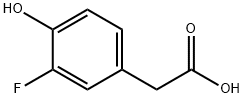
458-09-3
110 suppliers
$25.00/1g

444807-50-5
13 suppliers
inquiry
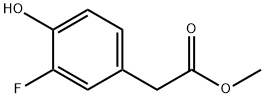
79280-92-5
17 suppliers
inquiry

444807-50-5
13 suppliers
inquiry
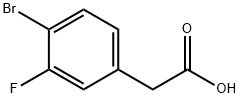
942282-40-8
102 suppliers
$18.14/1g

444807-50-5
13 suppliers
inquiry