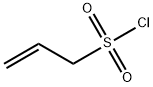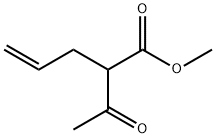
methyl 2-acetylpent-4-en-1-oate synthesis
- Product Name:methyl 2-acetylpent-4-en-1-oate
- CAS Number:3897-04-9
- Molecular formula:C8H12O3
- Molecular Weight:156.18
Yield:3897-04-9 96%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20; for 6 h;Tsuji-Trost Allylation;Reagent/catalyst;
Steps:
4.2 General Procedure for the Allylation/Cinnamylation of 1,3-Dicarbonyl CompoundsUsing MMZNiY Zeolite
General procedure: A mixture of 1,3-dicarbonyls (1 mmol), allyl/cinnamyl bromide(2 mmol) and K2CO3(1 mmol) were added to the reactiontube, which contains acetonitrile (3 ml) and 100 mg of freshly activated MMZNiYzeolite. The reaction mixturewas stirred for 6 h. Products were extracted from the reactionmixture after the addition of 15 ml of dichloromethanesolvent and allowed for 2 h stirring again. The catalyst wasseparated from the reaction mixture by simple filtration.Then, the solvents were removed by evaporation in a rotaryevaporator. Proceedings of the reaction was constantly monitoredby TLC [silica gel, pet ether:ethyl acetate (8:2)]. Theproducts were purified by column Chromatography [usingpet ether (97%):ethyl acetate (3%)].
References:
Senthilkumar, Samuthirarajan;Thangapriya, Cheirmakani;Alagumurugayee, Raman;Kumarraja, Mayilvasagam [Catalysis Letters,2017,vol. 147,# 11,p. 2755 - 2763]
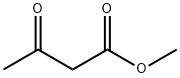
105-45-3
666 suppliers
$5.00/5g

107-18-6
2 suppliers
$24.60/100ml

3897-04-9
2 suppliers
inquiry

3666-84-0
1 suppliers
inquiry