METHYL 2-AMINO-5-PHENYL-1,3-THIAZOLE-4-CARBOXYLATE synthesis
- Product Name:METHYL 2-AMINO-5-PHENYL-1,3-THIAZOLE-4-CARBOXYLATE
- CAS Number:80625-18-9
- Molecular formula:C11H10N2O2S
- Molecular Weight:234.27

116-54-1
190 suppliers
$5.00/5G

100-52-7
945 suppliers
$21.30/2g

17356-08-0
3 suppliers
inquiry

80625-18-9
10 suppliers
inquiry
Yield:80625-18-9 70%
Reaction Conditions:
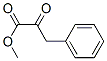
Stage #1: dichloroacetic acid methyl ester;benzaldehydewith sodium methylate in methanol;diethyl ether at 0; for 1 h;
Stage #2: thiourea in methanol; for 18 h;Heating / reflux;
Steps:
16
EXAMPLE 16 Preparation of Methyl 2-amino-5-phenyl-1,3-thiazole-4-carboxylate; [0111] EMI313.0[0112] A solution of NaOMe (25 wt %) in MeOH (13.4 mmol) was added to a solution of methyl dichloroacetate (13.4 mmol) and benzaldehyde (14.8 mmol, 1.1 eq) in Et2O (8 mL) dropwise at 0[deg.] C. The reaction mixture was stirred at 0[deg.] C. for 1 hour before Et2O and brine were added. The organic layer was separated, dried over anhyd. sodium sulfate, and solvent was removed in vacuo to give a crude material which was dissolved in MeOH (16 mL) containing thiourea (11.4 mmol, 0.85 eq). The resulting reaction mixture was heated to reflux for 18 hours. The crude product mixture was concentrated in vacuo, neutralized with 18M-NH4OH at which time the product precipitated as a white solid. The product was washed with CH2Cl2 (2*), water, and was collected by filtration to afford 1.88 g (70%) of product. MH<+>=235.1, Rf=0.18 (hexanes:EtOAc=1:1), retention time (LC-MS)=1.86 min.
References:
US2003/195352,2003,A1 Location in patent:Page/Page column 30; 34

100-52-7
945 suppliers
$21.30/2g

80625-18-9
10 suppliers
inquiry
32803-73-9
6 suppliers
inquiry

80625-18-9
10 suppliers
inquiry