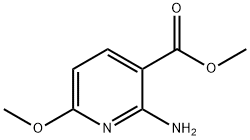
METHYL 2-AMINO-6-METHOXYPYRIDINE-3-CARBOXYLATE synthesis
- Product Name:METHYL 2-AMINO-6-METHOXYPYRIDINE-3-CARBOXYLATE
- CAS Number:1227048-93-2
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18
![3-Pyridinecarboxylic acid, 6-methoxy-2-[[(4-methoxyphenyl)methyl]amino]-, methyl ester](/CAS/20210111/GIF/1298123-87-1.gif)
1298123-87-1
0 suppliers
inquiry

1227048-93-2
24 suppliers
inquiry
Yield:1227048-93-2 97%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol at 27; for 16 h;Inert atmosphere;
Steps:
Preparation of methyl 2-amino-6-methoxynicotinate
To a stirred solution of methyl 6-methoxy-2-((4-methoxybenzyl) amino)nicotinate (16 g, 51.3 mmol) in EtOH (150 mL) at 27 °C under nitrogen atmosphere was added Pd-C(16.39 g, 15.40 mmol) portion-wise. The reaction mixture was purged with hydrogen gas and then stirred under balloon-pressure hydrogen atmosphere for 16 h. The progress of the reaction was monitored by TLC (Si02, 30% EtOAc/Pet., Rf 0.3, UV-active). Upon completion, the reaction mixture was filtered through a small pad of Celite and the filter cake was extracted with DCM (200 mL). The filtrate was concentrated under reducedpressure to obtain methyl 2-amino-6-methoxynicotinate (15 g, off-white solid). This procedure was repeated to afford a second batch of cmde product. Both batches were dissolved in EtOAc and blended. The solution was concentrated under reduced pressure to afford the combined cmde compound (32 g, 79 mmol). This material was purified by column chromatography on silica gel (Si02, 100-200 mesh) eluting with 0-30%EtOAc/Pet. The fractions containing product were collected and concentrated under reduced procedure to afford methyl 2-amino-6-methoxynicotinate (14 g, Yield: 97%, offwhite solid). ‘HNMR (400 MHz, DMSO-d6) 5 = 7.93 (d, J= 8.6 Hz, 1H), 7.28 (br s, 2H), 6.01 (d, J= 8.6 Hz, 1H), 3.82 (s, 3H), 3.76 (s, 3H). LCMS: RT = 1.89 mins, (M+H) =183.21, Purity = 99%.
References:
WO2020/95176,2020,A1 Location in patent:Page/Page column 65
38496-18-3
377 suppliers
$6.00/1g

1227048-93-2
24 suppliers
inquiry

503000-87-1
88 suppliers
$37.00/250mg

1227048-93-2
24 suppliers
inquiry

95652-77-0
92 suppliers
$82.00/100mg

1227048-93-2
24 suppliers
inquiry