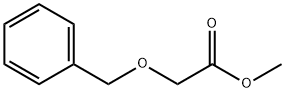
methyl 2-(benzyloxy)acetate synthesis
- Product Name:methyl 2-(benzyloxy)acetate
- CAS Number:31600-43-8
- Molecular formula:C10H12O3
- Molecular Weight:180.2

67-56-1
738 suppliers
$7.29/5ml-f
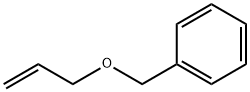
14593-43-2
122 suppliers
$18.00/1g

31600-43-8
65 suppliers
$45.00/100mg
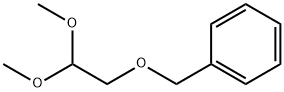
127657-97-0
66 suppliers
$26.00/5g
Yield: 56% , 42%
Reaction Conditions:
Stage #1:methanol;benzyl allyl ether with oxygen;ozone at -78;
Stage #2: with semicarbazide hydrochloride at -50 - 20; for 24 h;
Steps:
Ozonation of Allyl Aryl Ethers Ia and Ib
General procedure: (2) An ozone-oxygen mixture was bubbled through a solution of 10.00 mmol of ethers Ia or Ib in50 mL of MeOH at -78°C until blue color appeared.The reaction mixture was purged with argon, 3.88 g(35.00 mmol) of NH2C(O)NHNH2 HCl. HCl was added at -50°C. The mixture was stirred at ambient temperature for 24 h. The reaction mixture was evaporated, the residue was dissolved in 100 mL of CHCl3, washed with 2 (to pH ≈ 7), and dried with Na2SO4. After evaporation, ether I produced 1.86 g of a mixture of compounds II and IV in 40 : 60 ratio(according to GLC and NMR), column chromatography (Si2, PE, PE : MTBE = 5 : 1, 4 : 1) afforded0.74 g (42%) of ester IIa and 1.09 g (56%) of acetal IV.After evaporation, ether Ib produced 1.64 g of a mixture of compounds IIb and IVb in 25 : 75 ratio (according to GLC and NMR data), column chromatography (Si2, PE, PE : MTBE = 5 : 1, 4 : 1) gave 0.40 g (24%)of ester IIb and 1.15 g (63%) of acetal IVb.
References:
Raskil'dina;Legostaeva;Garifullina;Sultanova;Ishmuratov;Zlotskii [Doklady Chemistry,2015,vol. 462,# 1,art. no. 9489,p. 127 - 129][Dokl. Akad. Nauk,2015,vol. 462,# 3,p. 307 - 309,3]

67-56-1
738 suppliers
$7.29/5ml-f
30379-55-6
230 suppliers
$13.00/10g

31600-43-8
65 suppliers
$45.00/100mg

100-39-0
424 suppliers
$10.00/10 g

31600-43-8
65 suppliers
$45.00/100mg

67-56-1
738 suppliers
$7.29/5ml-f
19810-31-2
163 suppliers
$18.00/1g

31600-43-8
65 suppliers
$45.00/100mg

96-35-5
316 suppliers
$5.00/5g

100-39-0
424 suppliers
$10.00/10 g

31600-43-8
65 suppliers
$45.00/100mg