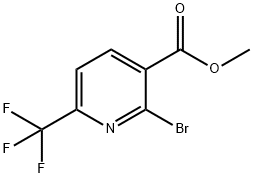
Methyl 2-bromo-6-(trifluoromethyl)nicotinate synthesis
- Product Name:Methyl 2-bromo-6-(trifluoromethyl)nicotinate
- CAS Number:144740-56-7
- Molecular formula:C8H5BrF3NO2
- Molecular Weight:284.03

67-56-1
757 suppliers
$7.29/5ml-f
191595-63-8
124 suppliers
$18.00/250mg

144740-56-7
40 suppliers
$323.00/1g
Yield: 42.1%
Reaction Conditions:
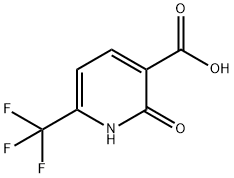
Stage #1:2-oxo-6-(trifluoromethyl)-1,2-dihydropyridine-3-carboxylic acid with pyridine;phosphorus(V) oxybromide in chlorobenzene at 20 - 120; for 16 h;
Stage #2:methanol in chlorobenzene for 1 h;
Steps:
d d) Methyl 2-bromo-6-(trifluoromethyl)nicotinate:
To the stirred solution of 2-hydroxy-6-(trifluoromethyl)nicotinic acid (23 g, 111 mmol) and pyridine (8.98 mL, 111 mmol) in chlorobenzene (250 mL) phosphorus oxybromide (63.7 g, 222 mmol) was added small portions wise at room temperature and the mixture was stirred at 120 °C for 16 h. After completion, the reaction mixture was concentrated under vacuum. The residue was cooled 0 °C and added excess cold methanol slowly. The solution stirred additional 1 h and again concentrated under vacuum. The residue dissolved in water and pH adjusted to ~8 using K2CO3 before extraction with EtOAc. The organic layer was separated and dried over anhydrous Na2S04, filtered and the filtrate was concentrated under reduced pressure to obtain as a brown liquid. The crude was purified by flash column chromatography on silica gel (100-200 mesh), eluting with 0-10% gradient of EtOAc in hexane to afford 4-bromo- 1 -(tetrahydro-2//-pyran-2- yl)-lf/-pyrazole in (400 g, 42.1%) yields. 1H NMR (500 MHz, CDCI3) d 8.20 (d, / = 8 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 4.01 (s, 3H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;BANDYOPADHYAY, Anish;CHEUNG, Mui;EIDAM, Hilary Schenck;JOSHI, Hemant;SU, Dai-Shi WO2019/149959, 2019, A1 Location in patent:Page/Page column 67; 69
1001924-23-7
7 suppliers
inquiry

144740-56-7
40 suppliers
$323.00/1g

116548-03-9
112 suppliers
$53.00/1g

144740-56-7
40 suppliers
$323.00/1g