
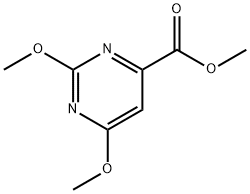
METHYL 2-CHLORO-6-METHOXYPYRIMIDINE-4-CARBOXYLATE synthesis
- Product Name:METHYL 2-CHLORO-6-METHOXYPYRIMIDINE-4-CARBOXYLATE
- CAS Number:127861-30-7
- Molecular formula:C7H7ClN2O3
- Molecular Weight:202.6

124-41-4
686 suppliers
$12.00/25g
6299-85-0
188 suppliers
$10.00/1g

127861-30-7
26 suppliers
$163.33/0.25g
Yield:127861-30-7 90%
Reaction Conditions:
in methanol at 20; for 0.25 h;Product distribution / selectivity;
Steps:
61.A
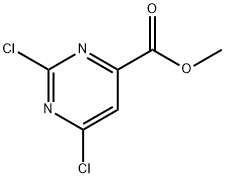
Example 61; N-hydroxy-2-(phenylamino)-6-(4-(pyridin-3-ylethynyl)phenyl)pyrimidine-4- carboxamide, trifluoroacetic acid salt; A. Methyl 2-chloro-6-methoxypyrimidine-4-carboxylate; To a solution of methyl 2,6-dichloropyrimidine-4-carboxylate (6.73 g, 32.51 mmol) in MeOH (5 mL) was added sodium methoxide, 0.5M solution in MeOH (58.5 mL, 29.26 mmol) and the reaction was stirred at room temperature. LC-MS after 15 minutes indicated reaction was complete. The reaction mixture was diluted with water and extracted three times with ethyl acetate. The combined organic extracts were dried over magnesium sulfate, filtered and evaporated to yield desired product as a white solid (5.9g, 90% yield). Regiochemistry was confirmed by NMR. LC-MS: [M+H]+ 203 Mass: calculated for C7H7ClN2O3, 202.60 IH NMR (300 MHz, DMSO-J6) δ ppm 3.90 (s, 3 H) 4.01 (s, 3 H) 7.45 (s, 1 H)
References:
WO2010/100475,2010,A1 Location in patent:Page/Page column 140-141

67-56-1
738 suppliers
$9.00/25ml

6299-85-0
188 suppliers
$10.00/1g

127861-30-7
26 suppliers
$163.33/0.25g

67-56-1
738 suppliers
$9.00/25ml

6299-85-0
188 suppliers
$10.00/1g

127861-30-7
26 suppliers
$163.33/0.25g
55878-45-0
30 suppliers
$200.00/50mg