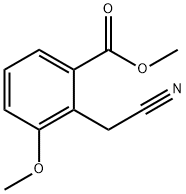
Methyl 2-(cyanoMethyl)-3-Methoxybenzoate synthesis
- Product Name:Methyl 2-(cyanoMethyl)-3-Methoxybenzoate
- CAS Number:145498-86-8
- Molecular formula:C11H11NO3
- Molecular Weight:205.21
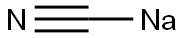
143-33-9
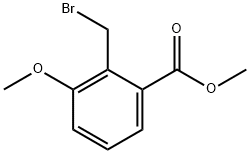
71887-28-0

145498-86-8
To a 500 mL three-necked round-bottomed flask were added 10 g (61.75 mmol) of methyl 2-bromomethyl-3-methoxybenzoate, 4.0 g (81.6 mmol) of sodium cyanide (NaCN), 0.30 g (2 mmol) of sodium iodide (NaI), 100 mL of acetonitrile (CH3CN) and 50 mL of N,N-dimethylformamide (DMF). The reaction mixture was heated to reflux and maintained for 10 hours. Upon completion of the reaction, the resulting sodium bromide (NaBr) precipitate was removed by filtration and the filtrate was concentrated by rotary evaporator. To the concentrate, 300 mL of water and 200 mL of ether were added and transferred to a dispensing funnel and shaken thoroughly. The aqueous phase was extracted twice with 100 mL of ether. The organic phases were combined, dried with anhydrous magnesium sulfate (MgSO4), filtered and concentrated to obtain methyl 3-methoxy-2-cyanomethylbenzoate in 95-100% yield. The above ester product (10.51 g, 0.053 mol) was dissolved in 100 mL of methanol (CH3OH), barium hydroxide octahydrate (Ba(OH)2-8H2O, 14.97 g, 0.079 mol) was added and stirred at room temperature overnight. After completion of the reaction, methanol was removed by rotary evaporation. To the residue was added 150 mL of water, 200 mL of dichloromethane (CH2Cl2) and 50 mL of 6N hydrochloric acid (HCl) and stirred until all solids were dissolved. The mixture was transferred to a separatory funnel and acidified with 6N hydrochloric acid to pH 1-2. The dichloromethane phase was separated and the aqueous phase was extracted twice with 50 mL of dichloromethane. The organic phases were combined, dried with anhydrous magnesium sulfate and activated charcoal, filtered and the solvent evaporated to give 8.8 g of white solid 2-cyanomethyl-3-methoxybenzoic acid in 87% yield. 1H NMR (CDCl3, 300 MHz) δ (ppm) of methyl 3-methoxy-2-cyanomethylbenzoate: 7.6 (d, 1H), 7.4 (t, 1H), 7.1 (d, 1H), 4.18 (s, 2H), 3.94 (s, 3H), 3.926 (s, 3H). tlc (1:1 ethyl acetate:hexane) Rf = 0.55 TLC (1:1 ethyl acetate:hexane) Rf = 0.55. 1H NMR (300 MHz, CDCl3) δ (ppm) of 2-cyanomethyl-3-methoxybenzoic acid: 7.55 (d, 1H), 7.45 (t, 1H), 7.3 (d, 1H), 4.121 (d, 2H), 3.91 (s, 3H). tlc (1:1 ethyl acetate:hexanes) Rf value not provided.
Yield: 95%
Reaction Conditions:
with sodium iodide in N,N-dimethyl-formamide;acetonitrile for 10 h;Heating / reflux;
Steps:
1.9 1.9
Into a 500 mL 3-neck round bottom flask was added 10 g (61.75 mmol) of 2-bromomethyl, 3-methoxy methyl benzoate, 4.0 g (81.6 mmol) of NaCN, 0.30 g (2 mmol) of NaI, 100 mL of CH3CN, and 50 mL of DMF. The reaction mixture was heated and refluxed for 10 hours. The precipitate (NaBr) was filtered off, and the solution was concentrated on an evaporator. 300 mL of water and 200 mL of ether were added and then shaken in a separatory funnel. The water was extracted twice with 100 mL of ether. The ether fractions were dried over MgSO4, and concentrated to yield methyl 3-methoxy-2-cyanomethylbenzoate (95-100% yield). This ester (0.053 mmol, 10.51 g) was stirred vigorously in 100 mL of CH3OH. Ba(OH)2 H2O (0.079 mmol, 14.97 g) was added and the mixture stirred at room temperature overnight. The CH3OH was removed on a rotary evaporator. 150 mL of water, 200 mL of CH2Cl2, and 50 mL of 6N HCl were added, and then stirred in a flask to dissolve all residues. The mixture was transferred to a separatory funnel, acidified with 6N HCl to pH 1-2. The CH2Cl2 phase was separated and the aqueous phase extracted twice with 50 mL of CH2Cl2. The CH2Cl2 extracts were combined, dried over MgSO4 and charcoal, filtered, and evaporated to yield 8.8 g of a white solid, 2-cyanomethyl-3-methoxybenzoic acid, (87%). Methyl 3-methoxy-2-cyanomethylbenzoate: 1H NMR (CDCl3, 300 MHz) δ (ppm): 7.6 (d, 1H), 7.4 (t, 1H), 7.1 (d, 1H), 4.18 (s, 2H), 3.94 (s, 3H), 3.926 (s, 3H). TLC (1:1 ethyl acetate: hexane) 0.55. 2-Cyanomethyl-3-methoxybenzoic acid: 1H NMR (300 Mhz, CDCl3) δ (ppm): 7.55 (d, 1H), 7.45 (t, 1H), 7.3 (d, 1H), 4.121 (d, 2H), 3.91, (s, 3H). TLC (1:1 ethyl acetate: hexane
References:
Hormann, Robert Eugene;Potter, David W.;Chortyk, Orestes;Tice, Colin M.;Carlson, Glenn Richard;Meyer, Andrew;Opie, Thomas R. US2006/20146, 2006, A1 Location in patent:Page/Page column 32