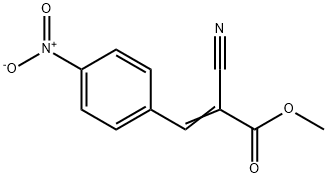
methyl (2E)-2-cyano-3-(4-nitrophenyl)prop-2-enoate synthesis
- Product Name:methyl (2E)-2-cyano-3-(4-nitrophenyl)prop-2-enoate
- CAS Number:62985-31-3
- Molecular formula:C11H8N2O4
- Molecular Weight:232.19
Yield:62985-31-3 92%
Reaction Conditions:
with C44H52CuN8S4(1+)*BF4(1-) in methanol at 70; for 4 h;Schlenk technique;Inert atmosphere;Knoevenagel Condensation;
Steps:
4.2 General Procedure for KnoevenagelCondensation Reactions
General procedure: In a typical procedure, aldehyde (1 mmol), active methylenegroup compound (1.2 mmol), and catalyst (5 mol%) wereplaced in a Schlenk tube, and then methanol (2 mL) wasadded. The reaction mixture was stirred for 4 h under aerobicconditions. The reaction progress was monitored by TLC.After completion of the reaction, water was added to formthe precipitate. The precipitate was filtered and re-dissolvedin ethyl acetate, followed by flash column chromatographyto remove the catalyst.
References:
Mannarsamy, Maruthupandi;Prabusankar, Ganesan [Catalysis Letters,2022,vol. 152,# 8,p. 2327 - 2332]

