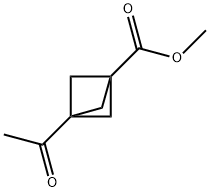
Methyl 3-acetylbicyclo[1.1.1]pentane-1-carboxylate synthesis
- Product Name:Methyl 3-acetylbicyclo[1.1.1]pentane-1-carboxylate
- CAS Number:131515-42-9
- Molecular formula:C9H12O3
- Molecular Weight:168.19
Yield:131515-42-9 76%
Reaction Conditions:
with copper (I) iodide in tetrahydrofuran at -78 - 0; for 2 h;Inert atmosphere;
Steps:
20.2 Step 2: Preparation of methyl 3-acetylbicyclo[1.1.1]pentane-1-carboxylate 20-2
MeLi (0.88 mL, 1.6 M in THF, 1.4 mmol) was added dropwise to a suspension of CuI (134 mg, 0.71 mmol) in anhydrous THF (2 mL) at 0° C. under a nitrogen atmosphere. The reaction mixture was cooled to -78° C., and a solution of compound 20-1 (110 mg, 0.59 mmol) in anhydrous THF (2 mL) was added dropwise. The reaction mixture was stirred at -78° C. for 2 hours. Methanol (0.6 mL) was added, and the resulting mixture was warmed up to room temperature. Saturated NH4Cl solution (10 mL) was added, and the resulting mixture was extracted with EtOAc (20 mL×3). The combined organic layer was washed with water and brine, dried over anhydrous Na2SO4, filtered and concentrated. The resulting residue was purified by column chromatography (silica gel, eluted with hexane/EtOAc from 5:1 to 2:1 (v/v)) to obtain compound 20-2 (75 mg, yield: 76%), as a pale yellow solid. MS (ESI): 169.1 [M+1]+1H NMR (400 MHz, CDCl3) δ: 3.62 (s, 3H), 2.24 (s, 6H), 2.07 (s, 3H)
References:
US2022/64113,2022,A1 Location in patent:Paragraph 0294; 0297-0299
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
144 suppliers
$24.00/100mg
![Methyl 3-acetylbicyclo[1.1.1]pentane-1-carboxylate](/CAS/20200331/GIF/131515-42-9.gif)
131515-42-9
25 suppliers
inquiry
![Bicyclo[1.1.1]pentane-1-carboxylic acid, 3-(chlorocarbonyl)-, methyl ester](/CAS/20210111/GIF/110371-22-7.gif)
110371-22-7
5 suppliers
inquiry
![Methyl 3-acetylbicyclo[1.1.1]pentane-1-carboxylate](/CAS/20200331/GIF/131515-42-9.gif)
131515-42-9
25 suppliers
inquiry
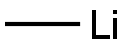

![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, 1-methyl 3-(2-thioxo-1(2H)-pyridinyl) ester](/CAS/20210305/GIF/156329-69-0.gif)