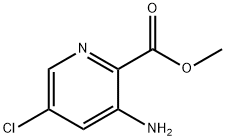
Methyl 3-aMino-5-chloropicolinate synthesis
- Product Name:Methyl 3-aMino-5-chloropicolinate
- CAS Number:866775-11-3
- Molecular formula:C7H7ClN2O2
- Molecular Weight:186.6

67-56-1
737 suppliers
$7.29/5ml-f
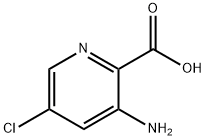
53636-68-3
61 suppliers
$45.00/50mg

866775-11-3
27 suppliers
inquiry
Yield:-
Reaction Conditions:
with thionyl chloride at 20 - 90; for 24 h;
Steps:
10.2
Step 2;. Preparation of 3-Amino-5-chloro-pyridine-2-carboxylic acid methyl ester; Heat at 90 °C a mixture of 5-chloro-3-nitro-pyridine-2-carbonitrile (1.0 g, 5.90 mmol) and tin (II) chloride (6.79 g, 29.5 mmol) in ethanol (10 mL) for 3 h. Evaporate the solvent under reduced pressure, add a solution of 35% hydrochloric acid (5 mL) and reflux the mixture for 6 h. Concentrate the reaction in vacuo to dryness and dissolve the resulting residue in methanol (20 mL). Add thionyl chloride (0.95 mL, 7.08 mmol) at room temperature and heat the mixture at 90 °C for 24 h. Remove the solvent under reduced pressure, add ethyl acetate, and wash with a saturated solution of sodium bicarbonate. Separate the organic layer, dry over sodium sulfate, filter, and concentrate under reduced pressure. Purify the residue using silica gel chromatography, eluting with ethyl acetate to afford the title compound (0.77 g, 70%). H-NMR (CDCl3, 3 00 MHz): 8 3.97 (s, 3 H) ; 5.85 (bs, 2 H) ; 7.06 (d, J = 2.0 Hz, 1H), 7.97 (d, J = 2.0 Hz, 1H).
References:
ELI LILLY AND COMPANY WO2005/97805, 2005, A1 Location in patent:Page/Page column 58

53636-68-3
61 suppliers
$45.00/50mg
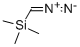
18107-18-1
206 suppliers
$33.00/5g

866775-11-3
27 suppliers
inquiry