
Methyl 3-aminobenzoate hydrochloride ,99% synthesis
- Product Name:Methyl 3-aminobenzoate hydrochloride ,99%
- CAS Number:87360-24-5
- Molecular formula:C8H10ClNO2
- Molecular Weight:188

67-56-1
739 suppliers
$9.00/25ml
99-05-8
512 suppliers
$14.00/5g

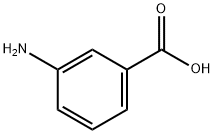
87360-24-5
26 suppliers
$45.00/10mg
Yield:87360-24-5 98%
Reaction Conditions:
with thionyl chloride for 16 h;
Steps:
1.41(i); 2
Compounds of Formula III; General Synthetic Scheme (Example - Compound 41); 41 (i)41 (iv) R = Boc -iDeprotection 41 R = H J TFA; Synthesis of Compound 41; 41(i); To a suspension of 3-aminobenzoic acid (1.03 g mg, 7.52 mmol) in MeOH (80 ml) at 0 °C was added dropwise thionyl chloride (5 ml). The resulting solution was allowed to stir for 16 h before the solvent was removed by evaporation and the product precipitated with diethyl ether. The diethyl ether was removed by evaporation to yield the title compound 41(i) as a white solid (1.38 g, 98%). Mp 176-178 °C.1H NMR (300 MHz, D2O) δ 3.66, s, 3H; 7.37, m, IH; 7.42, m, IH; 7.71, m, IH; 7.75, dt, J = 1.8, 3.3, 7.2 Hz, IH. MS (CI) m/z 152 (100%) [M]+; Methyl 3-amino-benzoate hydrochloride (143); To a suspension of 3-aminobenzoic acid (1.03 g mg, 7.52 mmol) in MeOH (80 mL) at O0C was added dropwise thionyl chloride (5 mL). The resulting solution was allowed to stir for 16 h before the solvent was removed by evaporation and the product precipitated with diethyl ether. The diethyl ether was removed by evaporation to yield the title compound (1.38 g, 7.38 mmol, 98%) as a white solid. Mp 176-1780C. 1H NMR (D20, 300 MHz): δ 7.75 (dt, J = 1.8, 3.3, 7.2 Hz, IH, ArH); 7.71 (m, IH, ArH); 7.42 (m, IH, ArH); 7.37 (m,lH, ArH); 3.66 (s, 3H, OCH3). Mass Spectrum (CI) m/z 152 (100%) [M+]. HRMS calcd for C8H10NO2 152.0712, found 152.0698..
References:
WO2006/74501,2006,A1 Location in patent:Page/Page column 124; 192

99-05-8
512 suppliers
$14.00/5g

87360-24-5
26 suppliers
$45.00/10mg