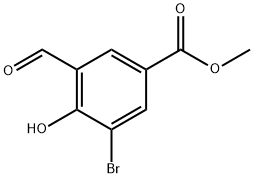
Methyl 3-broMo-5-forMyl-4-hydroxybenzoate synthesis
- Product Name:Methyl 3-broMo-5-forMyl-4-hydroxybenzoate
- CAS Number:706820-79-3
- Molecular formula:C9H7BrO4
- Molecular Weight:259.05

24589-99-9
140 suppliers
$11.00/250mg

706820-79-3
33 suppliers
$130.00/0.25G
Yield:706820-79-3 89%
Reaction Conditions:
with N-Bromosuccinimide in DMF (N,N-dimethyl-formamide) at 0 - 20; for 0.666667 h;
Steps:
16
A MIXTURE OF 4-HYDROXYMETHYLBENZOATE (64.8 G, 0.426 MOL, 1.0 EQ. ) AND ACETONITRILE (1 L) was stirred at room temperature while triethylamine (119 mL, 86.2 g, 0.852 mol, 2.0 EQ. ), MAGNESIUM CHLORIDE (60.8 G, 0.639 MOL, 1.5 EQ.) AND PARAFORMALDEHYDE (38.3 G, 1.28 MOL, 3.0 EQ. ) WERE ADDED SEQUENTIALLY. THE MIXTURE WAS HEATED AT 80 TO 82°C AND MONITORED by HPLC. Further quantities of magnesium chloride (20.3 g) and PARAFORMALDEHYDE (20 g) were added at 1.5 and 3.5 hours each. The mixture was heated a total of 19 hours and then concentrated. The residue was combined with ethyl acetate (500 mL) and 1M hydrochloric acid (1 L) added and the mixture stirred until a solution was obtained. The aqueous layer was separated and extracted with ethyl acetate (500 mL) and the combined organic phases were washed with 1M hydrochloric acid (500 ML) and then brine, dried (MGSO4) and concentrated. Purification of the product from the residue by filtration chromatography through silica gel (1000 mL), eluting first with DCM (3 x 1 L) and then 2% MEOH/DCM (3 x 1 L), gave methyl 3-formyl-4-hydroxy-benzoate (41.2 g, 54%) as a white solid. RP-HPLC (10-95S) RT = 3. 30 min ; 1H NMR (400 MHz, d6-DMSO): 8 11.54* (1H, s), 10.70 (1H, s), 8.22 (1H, d, J = 2.0 Hz), 8.03 (1H, dd, J = 8.0, 2.0 Hz), 7.08 (1H, dd, J = 8. 0,2. 0 Hz), 3.82 (3H, s); m/z (LCMS-ESI): Q-179 (M-H). [0211] A MIXTURE OF THE 3-FORMYL-4-HYDROXY-BENZOATE (41.2 G, 0.223 MOL, 1.0 EQ. ) AND N, N- DIMETHYLFORMAMIDE (225 ML) WAS COOLED TO 0°C AND N-BROMOSUCCINIMIDE (39.6 G, 1.0 EQ. ) was added in a single portion to the mixture. The mixture was cooled for 10 minutes and then allowed to stir at room temperature for 30 minutes. The mixture was poured into water (1.8 L), upon which a white precipitate formed. The precipitate was collected and washed with water (-2 L). Drying the damp solid azeotropically with toluene gave 3-bromo-5- FORMYL-4-HYDROXY-BENZOIC acid methyl ester (52.2 g, 89%) as an off-white solid. RP-HPLC (10-95S) RT = 4. 50 MIN.'H NMR (400 MHz, d6-DMSO): 8 10. 12 (1H, s), 8.32 (1H, d, J = 2.0 Hz), 8.28 (1H, d, J = 2.0 Hz), 3.85 (3H, s). [0212] A mixture OF 3-BROMO-5-FORMYL-4-HYDROXY-BENZOIC acid methyl ester (26.0 g, 100 mmol, 1.0 eq) and N, N DIMETHYLFORMAMIDE (400 mL) was stirred at room temperature WHILE POTASSIUM CARBONATE (20.73 G, 150 MMOL, 1.5 EQ. ) AND THEN BENZYL BROMIDE (15 ML, 21.6 G, 125 MMOL, 1.25 EQ. ) IN A SINGLE PORTION WAS ADDED TO THE MIXTURE. THE REACTION mixture was stirred for 24 hours and then poured into water (1 L). The precipitate was collected by filtration and the filtrate extracted with diethyl ether (x 3). The precipitate was dissolved with the ether extracts and the solution was washed with water and then brine, dried (MGSO4) and concentrated. Purifiction of product from the residue via filtration chromatography on a silica gel plug (1 L), loaded in 25% DCM/Hexane and eluted successively with 1 L portions of 25 % DCM/Hexane (x 2), then 50 % DCM/HEXANE (x 1) and finally 100% DCM (x 3), gave methyl 4-BENZYLOXY-3-BROMO-5-FORMYL-BENZOATE (25.2 g, 72%) as an off-white solid. RP-HPLC (10-95S) RT = 4.47 min ; 1H NMR (400 MHz, d6- DMSO): 8 10.00 (1H, s), 8.42 (1H, d, J = 2.0 Hz), 8.21 (1H, d, J = 2.0 Hz), 3.88 (3H, s); M/Z (LCMS-ESI): Q+ 371/373 (M+Na).
References:
WO2004/62661,2004,A1 Location in patent:Page/Page column 35-36
100-97-0
0 suppliers
$14.00/250G
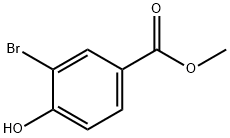
29415-97-2
171 suppliers
$5.00/1g

706820-79-3
33 suppliers
$130.00/0.25G
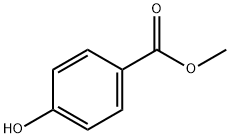
99-76-3
812 suppliers
$5.00/25g

706820-79-3
33 suppliers
$130.00/0.25G