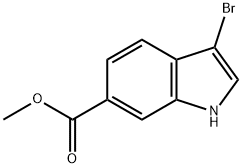
METHYL 3-BROMOINDOLE-6-CARBOXYLATE synthesis
- Product Name:METHYL 3-BROMOINDOLE-6-CARBOXYLATE
- CAS Number:860457-92-7
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
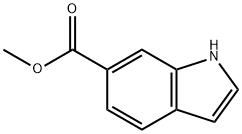
50820-65-0
250 suppliers
$6.00/1g

860457-92-7
66 suppliers
$45.00/100mg
Yield: 98%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at -60 - 20; for 3.33 h;Inert atmosphere;
Steps:
7 methyl 3-bromo-1H-indole-6-carboxylate (131-1).
Under N2 atmosphere, to an anhydrous DMF solution (10 mL) of methyl 1H-indole-6-carboxylate (1.00 g, 5.71 mmol) was added dropwise an anhydrous DMF (10 mL) solution of NBS (1.04 g, 5.84 mmol) at -60 oC over 20 min, and stirred and slowly warmed to rt in 3 h. The reaction mixture was worked up by ethyl acetate (100 mL) and water (20 mL, X3), and ethyl acetate layer was concentrated in vacuo and residue was purified by flash chromatography on silica gel with hexanes and ethyl acetate (EA: 0-40%) to give product (1.41 g, 98%).1H NMR (600 MHz, CD3OD) δ 8.15 (s, 1H), 7.80 (d, J = 6.0 Hz, 1H), 7.50 (m, 2H), 3.94 (s, 3H).
References:
THE REGENTS OF THE UNIVERSITY OF MICHIGAN;GREMBECKA, Jolanta;CIERPICKI, Tomasz;ROGAWSKI, David;BORKIN, Dmitry;KLOSSOWSKI, Szymon;ZHUANG, Jin;MONTGOMERY, Deanna;DENG, Jing;SZEWCZYK, Marta;LI, Hao WO2017/197240, 2017, A1 Location in patent:Page/Page column 204