
methyl 3-chloro-4-ethylbenzoate synthesis
- Product Name:methyl 3-chloro-4-ethylbenzoate
- CAS Number:1081551-72-5
- Molecular formula:C10H11ClO2
- Molecular Weight:198.65
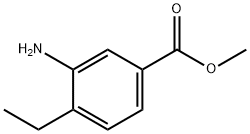
24812-93-9
16 suppliers
$425.00/1g

1081551-72-5
9 suppliers
inquiry
Yield:1081551-72-5 38%
Reaction Conditions:
Stage #1: 3-amino-4-ethylbenzoic acid methyl esterwith sulfuric acid;acetic acid;sodium nitrite at 0 - 5; for 1.5 h;
Stage #2: with hydrogenchloride;copper(l) chloride in water at 0; for 2 h;
Steps:
95.A A. methyl 3-chloro-4-ethylbenzoate
A. methyl 3-chloro-4-ethylb [00693] Charge NaN02 (579 mg, 8.37 mmol) and concentrated sulfuric acid (6 mL) to a flask. As methyl 3-amino-4-ethylbenzoate (1.37 g, 7.62 mmol, PREPARATION 5) in acetic acid (18 mL) was added at 0°C, color of the mixture turned to yellow, and then stirred at 5°C for 1.5 h. The solution above was added to a dark mixture of CuCl (1.65 g, 16.65 mmol) in concentrated HC1 (18 mL) slowly at 0°C, stirred for 2 h, and poured into ice water, extracted with DCM, the organic phase was concentrated to give 582 mg of the title compound (38%). 1H NMR (400 MHz, CDC13): δ 1.25 (3H, t, J= 8.0 Hz), 2.80 (2H, q, J= 7.6 Hz), 3.91 (3H, s), 7.30 (1H, d, J= 7.6 Hz), 7.85 (1H, dd, J= 1.2, 7.6 Hz), 8.01 (1H, d, J= 1.6 Hz).
References:
WO2014/89364,2014,A1 Location in patent:Paragraph 00692; 00693
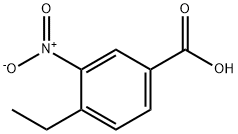
103440-95-5
92 suppliers
$10.00/1g

1081551-72-5
9 suppliers
inquiry
619-64-7
262 suppliers
$6.00/10g

1081551-72-5
9 suppliers
inquiry

51885-79-1
15 suppliers
$45.00/25mg

1081551-72-5
9 suppliers
inquiry