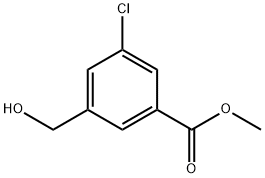
methyl 3-chloro-5-(hydroxymethyl)benzoate synthesis
- Product Name:methyl 3-chloro-5-(hydroxymethyl)benzoate
- CAS Number:153203-58-8
- Molecular formula:C9H9ClO3
- Molecular Weight:200.62
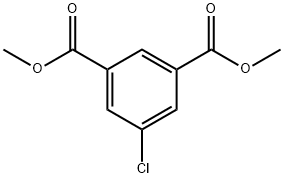
20330-90-9
91 suppliers
$45.00/50mg

153203-58-8
34 suppliers
$130.00/50mg
Yield:153203-58-8 66.9%
Reaction Conditions:
with sodium tetrahydroborate in methanol;dichloromethane at 0 - 25; for 16 h;
Steps:
Methyl 3-chloro-5-(hydroxymethyl)benzoate
Dimethyl 5-chloroisophthalate (90.0 g, 394 mmol, 1.00 eq) was dissolved in MeOH (900 mL) and DCM (900 mL) and the solution was cooled to 0 °C. NaBFL (59.6 g, 1.57 mol, 4.00 eq) was added in portions to the mixture. The reaction mixture was stirred at 25 °C for 16 hrs. TLC (petroleum ether / ethyl acetate = 3:1, Rf= 0.17) indicated ca. 30% of the starting compound remained, and one new spot was formed. The reaction mixture was poured into aqueoussaturated solutionof NH4CI (500 mL) and extracted with DCM (2x200 mL). The combined organic phases were washed with brine (500 mL), dried with anhydrous Na2SC>4, filtered and concentrated in vacuum. The residue was purified by column chromatography (S1O2, petroleum ether / ethyl acetate = 50/1 to 3/1). Methyl 3-chloro-5-(hydroxymethyl)benzoate (52.8 g, 263 mmol, 66.9% yield) was obtained as colorless oil.
References:
WO2021/165195,2021,A1 Location in patent:Page/Page column 126

153203-57-7
26 suppliers
inquiry

153203-58-8
34 suppliers
$130.00/50mg