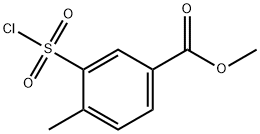
methyl 3-(chlorosulfonyl)-4-methylbenzoate synthesis
- Product Name:methyl 3-(chlorosulfonyl)-4-methylbenzoate
- CAS Number:372198-41-9
- Molecular formula:C9H9ClO4S
- Molecular Weight:248.68

67-56-1
739 suppliers
$9.00/25ml
2548-29-0
73 suppliers
$24.00/1g

372198-41-9
25 suppliers
$170.00/500mg
Yield:372198-41-9 60%
Reaction Conditions:
Stage #1: 3-(chlorosulfonyl)-4-methylbenzoic acidwith thionyl chloride for 1 h;Reflux;
Stage #2: methanol for 1 h;
Steps:
4.1.3.9. Methyl 3-(2-((5-cyanopyrazolo[1,5-a]pyridin-3-yl)methylene)-1-methylhydrazinylsulfonyl)-4-methylbenzoate (6i)
3-(Chlorosulfonyl)-4-methylbenzoic acid (200 mg, 0.85 mmol) was refluxed in SOCl2 (1.0 mL) for 1 h. The solvent was removed in vacuo, then MeOH (5 mL) added and the solution stirred for 1 h. The solvent was removed in vacuo to leave methyl 3-(chlorosulfonyl)-4-methylbenzoate as a pale brown solid (127 mg, 60%). 1H NMR δ (400 MHz, CDCl3) 8.72 (d, J = 1.7 Hz, 1H), 8.25 (dd, J = 7.9, 1.7 Hz, 1H), 7.52 (d, J = 7.9 Hz, 1H), 3.97 (s, 3H), 2.86 (s, 3H). LC-MS (APCI-) 229 (M-Cl+O, 100%). Reaction of 2 (30 mg, 0.18 mmol) and the above sulfonyl chloride (87 mg, 0.35 mmol) using NaHCO3, after chromatography (eluting with hexanes:EtOAc 2:1 to 1:1 to EtOAc) gave 6i as a yellow solid (53 mg, 74%).
References:
Kendall, Jackie D.;Giddens, Anna C.;Tsang, Kit Yee;Frédérick, Rapha?l;Marshall, Elaine S.;Singh, Ripudaman;Lill, Claire L.;Lee, Woo-Jeong;Kolekar, Sharada;Chao, Mindy;Malik, Alisha;Yu, Shuqiao;Chaussade, Claire;Buchanan, Christina;Rewcastle, Gordon W.;Baguley, Bruce C.;Flanagan, Jack U.;Jamieson, Stephen M.F.;Denny, William A.;Shepherd, Peter R. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 58 - 68] Location in patent:experimental part
99-75-2
268 suppliers
$6.00/10g

372198-41-9
25 suppliers
$170.00/500mg
547739-67-3
6 suppliers
inquiry

99-75-2
268 suppliers
$6.00/10g

372198-41-9
25 suppliers
$170.00/500mg
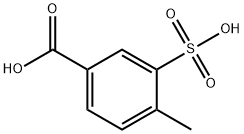
62454-72-2
0 suppliers
inquiry

99-94-5
567 suppliers
$9.00/1g

372198-41-9
25 suppliers
$170.00/500mg