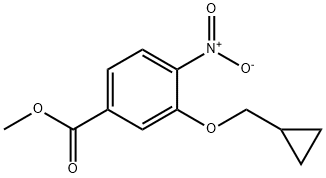
METHYL 3-(CYCLOPROPYLMETHOXY)-4-NITROBENZOATE synthesis
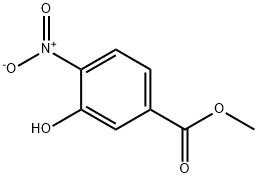
- Product Name:METHYL 3-(CYCLOPROPYLMETHOXY)-4-NITROBENZOATE
- CAS Number:1239278-72-8
- Molecular formula:C12H13NO5
- Molecular Weight:251.24
Yield: 100%
Reaction Conditions:
with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 50;Inert atmosphere;
Steps:
20.2 Step 2:
Step 2:
Synthesis of methyl 3-(cyclopropylmethoxy)-4-nitrobenzoate (179)
Methyl 3-hydroxy-4-nitrobenzoate (5.53 g, 28.1 mmol) was dissolved in dry DMF (65 ml) under N2 atmosphere.
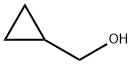
Potassium iodide (1.397 g, 8.42 mmol), potassium carbonate (12.79 g, 93 mmol) and then (bromomethyl)cyclopropane (5.44 ml, 56.1 mmol) were added.
The reaction mixture was vigorously stirred at 50° C. overnight.
The solvent was evaporated and the residue was treated with AcOEt and water.
The aqueous phase was extracted with AcOEt and the combined organic layer was dried over Na2SO4 and concentrated to give the desired product as a yellow solid (7 g, 100% yield). MS/ESI+ 252 [MH]+
References:
CHIESI FARMACEUTICI S.p.A.;ARMANI, Elisabetta;Amari, Gabriele;Esposito, Oriana;Carzaniga, Laura;Capaldi, Carmelida US2014/155391, 2014, A1 Location in patent:Paragraph 0536; 0538
619-14-7
292 suppliers
$10.00/5g

1239278-72-8
8 suppliers
inquiry