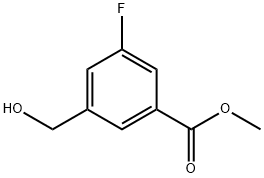
Methyl 3-Fluoro-5-(hydroxymethyl)benzoate synthesis
- Product Name:Methyl 3-Fluoro-5-(hydroxymethyl)benzoate
- CAS Number:660416-37-5
- Molecular formula:C9H9FO3
- Molecular Weight:184.16

17449-48-8
41 suppliers
$12.00/250mg

660416-37-5
16 suppliers
inquiry
Yield:660416-37-5 57%
Reaction Conditions:
Stage #1: dimethyl 5-fluoroisophthalatewith lithium tetrahydridoborate in tetrahydrofuran at 0 - 20; for 3 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water monomer;
Steps:
6-24.6.1
5-Fluoroisophthalic acid dimethylester (1.6 g, 7.54 mmol) (J. Org. Chem ; 1969,34, 1960-1961) WAS dissolved in tetrahydrofuran (15 ml). Thereafter, therein 2.0 M lithiumborohydride tetrahydrofuran solution (2.6 ml, 5.27 mmol) was slowly added dropwise at 0°C, and the reaction mixture was refluxed for 3 hours. The reaction mixture was acidified with IN HC1 aqueous solution, concentrated under reduced pressure to remove solvent, and extracted with chloroform. Combined organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography (dichloromethane: METHANOL = 99 : 1), to give 800 mg (yield: 57%, white solid) of the target compound. 1H NMR (400MHZ, CDCl3) : 63. 92 (D, J=1. 6Hz, 3H), 4.75 (d, J=4Hz, 2H), 7.29-7. 32 (m, 1H), 7.61 (dd, J=9. 2, 1. 6Hz, 1H), 7.8 (d, J=0. 8Hz, 1H)
References:
WO2004/113281,2004,A1 Location in patent:Page 61-62