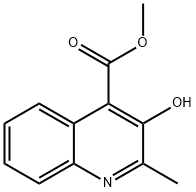
Methyl 3-hydroxy-2-methylquinoline-4-carboxylate synthesis
- Product Name:Methyl 3-hydroxy-2-methylquinoline-4-carboxylate
- CAS Number:104179-54-6
- Molecular formula:C12H11NO3
- Molecular Weight:217.22
Yield:104179-54-6 24%
Reaction Conditions:
with thionyl chloride at -10; for 20 h;Heating / reflux;
Steps:
22
EXAMPLE 22Synthesis of3-hydroxy-2-methylquιnohne-4-carbaldehydeO[203] 2-methyl-3-hydroxyqumolme-4-carboxyhc acid (1.016 g, 5 mmol) was dissolved in 10 mL methanol. Thionyl chloride (730 μL, 10 mmol) was added at -100C, and the mixture was heated at reflux for 20 h, with additions of 365 μL thionyl chloride (5 mmol) every 4 h The reaction mixture was evaporated, taken up m satd. sodium bicarbonate and the mixture was extracted with ethyl acetate The organic layer was evaporated and the crude product recrystallized from hexane to give 22a (258 mg, 1 1 mmol, 24%),
References:
WO2008/154484,2008,A1 Location in patent:Page/Page column 139

