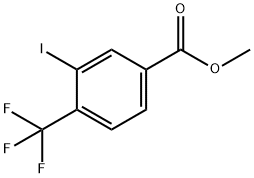
methyl 3-iodo-4-(trifluoromethyl)benzoate synthesis
- Product Name:methyl 3-iodo-4-(trifluoromethyl)benzoate
- CAS Number:1197357-94-0
- Molecular formula:C9H6F3IO2
- Molecular Weight:330.04
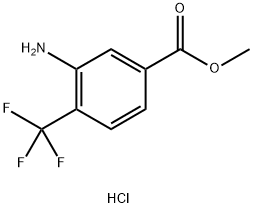
1197357-93-9
0 suppliers
inquiry

1197357-94-0
22 suppliers
inquiry
Yield:1197357-94-0 90%
Reaction Conditions:
Stage #1: methyl 3-amino-4-(trifluoromethyl)benzoate hydrochloride saltwith hydrogenchloride;sodium nitrite in water at -5 - 0; for 0.583333 h;
Stage #2: with potassium iodide;iodine in water at -5 - 90;
Steps:
19.2
A solution of sodium nitrite (1.34 g 19.3 mmol) in water (7.0 mL) was added dropwise to a rapidly stirred suspension of methyl 3-amino-4-(trifluoromethyl)benzoate, HCI salt (4.5 g, 17.5 mmol) from step 1 in 6 N aqueous HCI (11 mL) at -5 to 0 0C over a period of five min. After the reaction was stirred at -5 0C for 30 min., a solution of potassium iodide (2.9 g, 17.5 mmol) in water (6.0 mL) and a small crystal of iodine were added slowly to the diazonium chloride formed in the reaction suspension. The resulting dark red solution was allowed to warm to room temperature and heated at 90 0C for one hour. The reaction mixture was extracted with ethyl acetate. The collected ethyl acetate extracts were washed with water. Separation and evaporation afforded
References:
WO2009/143404,2009,A1 Location in patent:Page/Page column 138-139
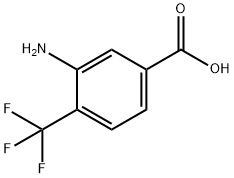
125483-00-3
82 suppliers
$10.00/250mg

1197357-94-0
22 suppliers
inquiry