
methyl 3-(methylamino)benzoate synthesis
- Product Name:methyl 3-(methylamino)benzoate
- CAS Number:104542-38-3
- Molecular formula:C9H11NO2
- Molecular Weight:165.19

168162-31-0
8 suppliers
inquiry

104542-38-3
8 suppliers
inquiry
Yield:104542-38-3 85%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 12 h;
Steps:
3 4.4.6.3. Methyl 3-(methylamino)benzoate (13)
Into a solution of 12(530 mg, 2 mmol) in dry CH2Cl2 (10 mL) was added trifluoroacetic acid (1 mL) at 0 C. The mixture was stirred at room temperature for 12 h, then the reaction was quenched with sat. NaHCO3 andthe whole was extracted with CH2Cl2. The combined organic layerswere washed with water and dried over anhydrous Na2SO4. Thesolvent was removed in vacuo and the residue was purified by flash chromatography on silica gel to give compound 13 as a colorlessoil. (280 mg, yield 85.0%). 1H NMR (400 MHz, DMSO-d6) d 7.18 (t,J = 7.7 Hz, 1H), 7.10 (d, J = 7.7 Hz, 2H), 6.77-6.72 (m, 1H), 5.98-5.90 (m, 1H), 3.77 (s, 3H), 2.66 (d, J = 5.1 Hz, 3H). ESI-MS: calcdfor [M+H]+ m/z 166.1, found: 166.2.
References:
Gao, Dingding;Li, Yingxia [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 14,p. 3780 - 3791]
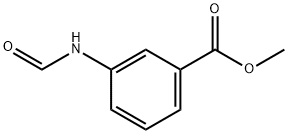
70531-23-6
0 suppliers
inquiry

104542-38-3
8 suppliers
inquiry
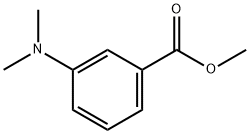
16518-64-2
51 suppliers
$45.00/100mg

104542-38-3
8 suppliers
inquiry
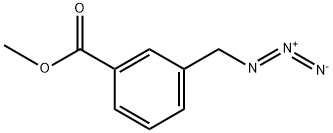
180863-54-1
1 suppliers
inquiry

104542-38-3
8 suppliers
inquiry

4518-10-9
191 suppliers
$12.80/1g

64-18-6
1107 suppliers
$22.12/250ML

104542-38-3
8 suppliers
inquiry