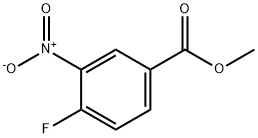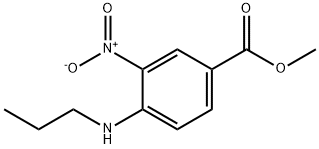
Methyl 3-nitro-4-(propylaMino)benzoate synthesis
- Product Name:Methyl 3-nitro-4-(propylaMino)benzoate
- CAS Number:128429-03-8
- Molecular formula:C11H14N2O4
- Molecular Weight:238.24
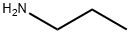
107-10-8
353 suppliers
$10.00/20mg
14719-83-6
212 suppliers
$5.00/1g

128429-03-8
8 suppliers
inquiry
Yield:128429-03-8 97.4%
Reaction Conditions:
with anhydrous Sodium acetate in water monomer;acetonitrile; for 17 h;Heating / reflux;
Steps:
11
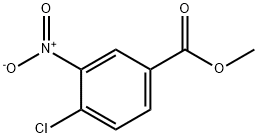
Methyl 4-chloro-3-nitrobenzoate (80.0 g, 0.371 mol) was dissolved in recently distilled acetonitrile (400 ml_) under stirring. Sodium acetate (61.0 g, 0.744 mol) and then propylamine (32.49 g, 0.55 mol) and distilled water (30 mL) were added to this solution under vigorous stirring, and the obtained mixture was refluxed for 17 h, during which time the course of the reaction was monitored by TLC (dichloromethane). The yellow reaction mixture was concentrated under reduced pressure to remove MeCN (200 mL). The residue was cooled and diluted with cold water (1.5 L). The obtained yellow precipitate was triturated in aqueous suspension, separated by filtration, washed with cold water (2 x 500 mL) and dried to give the title compound as bright yellow powder in 86.1 g, (97.4% ) yield.
References:
WO2007/69053,2007,A1 Location in patent:Page/Page column 28

96-99-1
491 suppliers
$5.00/5g

128429-03-8
8 suppliers
inquiry
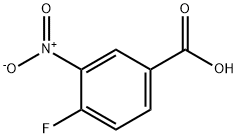
453-71-4
326 suppliers
$5.00/1g

128429-03-8
8 suppliers
inquiry