
METHYL 3-(OXETAN-3-YL)BENZOATE synthesis
- Product Name:METHYL 3-(OXETAN-3-YL)BENZOATE
- CAS Number:1044507-47-2
- Molecular formula:C11H12O3
- Molecular Weight:192.21
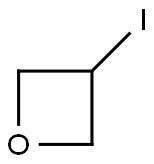
26272-85-5
233 suppliers
$10.00/1g
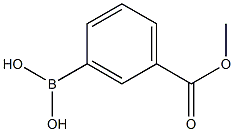
99769-19-4
263 suppliers
$10.00/1g

1044507-47-2
5 suppliers
inquiry
Yield:1044507-47-2 27%
Reaction Conditions:
Stage #1: 3-iodooxetane;[3-(methoxycarbonyl)phenyl]boronic acidwith potassium carbonate in 1,4-dioxane at 20 - 27; for 0.333333 h;Inert atmosphere;
Stage #2: with nickel(II) nitrate hexahydrate;4,4'-di-tert-butyl-2,2'-bipyridine in 1,4-dioxane at 80; for 4 h;Inert atmosphere;
Steps:
Step (i)
(3-(Methoxycarbonyl)phenyl)boronic acid (2.0 g, 11.07 mmol), 3-iodooxetane (4.07 g, 22.1 mmol) and K2CO3 (4.58 g, 33.2 mmol) were dissolved in dry 1 ,4-dioxane (10 mL). Argon gas was purged through the mixture at room temperature for 20 min, then Ni(NO3)2 hexahydrate (0.161 g, 0.55 mmol) and 4,4'-di-tert-butyl-2,2'-dipyridyl (0.14 g, 0.55 mmol) were added and the reaction mixture was heated to 80 °C for 4 hrs. The reaction mixture was then partitioned between water (250 mL) and EtOAc (250 mL). The aqueous layer was further extracted with EtOAc (2 X 150 mL). Organic layers were combined and dried (Na2SO4), the solvent was removed in vacuo and the crude product was purified by reverse phase gradient flash column chromatography (reverse phase, C18 silica), product eluted at 0% to 46% ACN in water to afford pure methyl 3-(oxetan-3-yl) benzoate (0.58 g, 27%) as a colourless liquid. 1 H NMR (400 MHz, DMSO-d6) d 3.86 (s, 3H), 4.33 (tt, J = 8.3, 6.6 Hz, 1H), 4.60 (dd, J = 6.6, 6.0 Hz, 2H), 4.97 (dd, J = 8.3, 6.0 Hz, 2H), 7.54 (t, J = 7.7 Hz, 1 H), 7.71 (dt, J = 7.7, 1 .3 Hz, 1 H), 7.87 (dt, J = 7.7, 1 .4 Hz, 1 H), 7.98 (t, J = 1.8 Hz, 1 H).
References:
WO2021/69927,2021,A1 Location in patent:Page/Page column 65-66