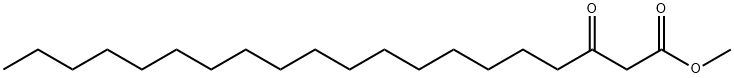
methyl 3-oxoicosanoate synthesis
- Product Name:methyl 3-oxoicosanoate
- CAS Number:14531-35-2
- Molecular formula:C21H40O3
- Molecular Weight:340.5405

67-56-1
739 suppliers
$9.00/25ml
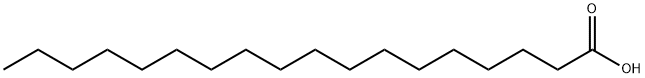
57-11-4
1210 suppliers
$6.00/100g

14531-35-2
4 suppliers
$106.00/25MG
Yield:14531-35-2 87%
Reaction Conditions:
Stage #1: cycl-isopropylidene malonate;stearic acidwith diethyl cyanophosphonate;triethylamine in dichloromethane at 20; for 16 - 18 h;
Stage #2: methanol for 4 - 16 h;Heating / reflux;
Steps:
47
Example 47 Preparation of methyl 3-oxoicosanoate (XXI) Stearic acid (5.0 g, 17.6 mmoles) is suspended in methylene chloride (88 mL) and 2,2-dimethyl-1,3-dioxane-4,6-dione (2.7 g, 18.5 mmoles), triethylamine (6.7 mL, 48.0 mmoles) and diethyl cyanophosphonate (2.7 mL, 17.6 mmoles) are added. All solids dissolve quickly. The solution is stirred for 16 hours at ambient temperature. The reaction mixture is then diluted with methylene chloride (100 mL) and carefully washed three times with aqueous hydrochloric acid (3 M, 50 mL each), three times with water (50 mL each) and once with saturated aqueous sodium chloride (50 mL). The reaction mixture is then dried over anhydrous sodium sulfate, filtered and evaporated. The resulting syrup is dissolved in anhydrous methanol (100 mL) and heated to reflux for 16 hours. The methanol is evaporated and the crude product dissolved in hexanes with slight heating. The crude product was then loaded on silica gel (150 mL bed volume) eluding with hexanes (500 mL) then hexane:acetone (98:2 [v/v], 1000 mL). Fractions containing pure product by thin layer chromatography are pooled and evaporated to dryness. The resulting oil is dried well in vacuo. The yield is 5.2 g (87%).
References:
US2008/85869,2008,A1 Location in patent:Page/Page column 31-32
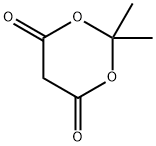
2033-24-1
637 suppliers
$6.00/25g
112-76-5
243 suppliers
$40.32/25G

14531-35-2
4 suppliers
$106.00/25MG

67-56-1
739 suppliers
$9.00/25ml

2033-24-1
637 suppliers
$6.00/25g

112-76-5
243 suppliers
$40.32/25G

14531-35-2
4 suppliers
$106.00/25MG

57-11-4
1210 suppliers
$6.00/100g

14531-35-2
4 suppliers
$106.00/25MG

67-56-1
739 suppliers
$9.00/25ml

2033-24-1
637 suppliers
$6.00/25g

57-11-4
1210 suppliers
$6.00/100g

14531-35-2
4 suppliers
$106.00/25MG