
METHYL 4-(3-FORMYL-2-PYRIDINYL)BENZENECARBOXYLATE synthesis
- Product Name:METHYL 4-(3-FORMYL-2-PYRIDINYL)BENZENECARBOXYLATE
- CAS Number:885950-15-2
- Molecular formula:C14H11NO3
- Molecular Weight:241.24
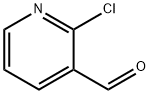
36404-88-3
314 suppliers
$5.00/1g
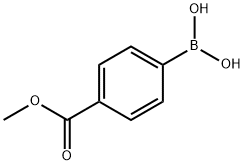
99768-12-4
358 suppliers
$10.00/1g

885950-15-2
19 suppliers
$373.00/1g
Yield:885950-15-2 77%
Reaction Conditions:
with sodium hydrogencarbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,2-dimethoxyethane;water at 75; for 2 h;
Steps:
14
Method 14; Preparation of methyl 4-(3-formylpyridin-2-yl)benzoate; To a solution of 2-chloronicotinaldehyde (1.58 g, 11.17 mmol) and (4-methoxycarbonylphenyl)boronic acid (2.0 g, 11.11 mmol) in 1,2-dimethoxyethane (75 ml) was added 1,1'-bis(diphenylphosphino)ferrocenedichloropalladium(II) dichloromethane adduct (432 mg, 0.53 mmol). The mixture was then purged with nitrogen for 5 minutes before the addition of saturated aqueous sodium bicarbonate solution (30 ml). The reaction mixture was heated to 75° C. with stirring for 2 hours, before cooling back to ambient temperature. The mixture was partitioned between ethyl acetate (200 ml) and water (200 ml). The organic layer was separated, washed with brine, dried over magnesium sulphate, filtered and evaporated to dryness. The resultant solid was purified by flash chromatography on silica, eluting with ethyl acetate and isohexane (1:1 v/v) followed by trituration under isohexane and diethyl ether to afford the title compound (2.07 g, 77%); NMR Spectrum: (DMSO-d6) δ 3.91 (s, 3H), 7.67 (m, 1H), 7.80 (d, 2H), 8.12 (d, 2H), 8.32 (m, 1H), 8.94 (m, 1H), 9.98 (s, 1H); Mass Spectrum: M+H+242.
References:
US2008/119451,2008,A1 Location in patent:Page/Page column 38