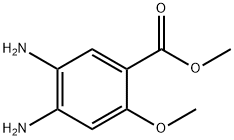
methyl 4,5-diamino-o-anisate synthesis
- Product Name:methyl 4,5-diamino-o-anisate
- CAS Number:59338-85-1
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2032
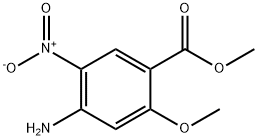
59338-84-0
79 suppliers
$75.00/250mg

59338-85-1
13 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol at 50; for 12 h;
Steps:
4
Step 4: A mixture of methyl 4-amino-2-methoxy-5-nitrobenzoate (3.0 g, 13.3 mmol) and Pd/C (0.3 g) in methanol (50 mL) was stirred under hydrogen at 50 °C for 12 h. After Pd/C was filtered, the filtrate was concentrated to afford methyl 4,5-diamino-2-methoxybenzoate as a brown solid (2.6 g, 99%). LC/MS (ES+): m/z calculated for C9H12N2O3: 196.1 ; found: 197.1 [M+H] NMR (400 MHz, CDCb): d 7.33 (s, 1H), 6.29 (s, 1H), 3.98 (br, 2H), 3.83 (s, 6H), 3.02 (br, 2H).
References:
KINETA, INC.;GOLDBERG, Daniel R.;PROBST, Peter;BEDARD, Kristin M. WO2020/36574, 2020, A1 Location in patent:Page/Page column 81-82
4093-29-2
224 suppliers
$5.00/250mg

59338-85-1
13 suppliers
inquiry
4093-41-8
50 suppliers
$115.00/5g

59338-85-1
13 suppliers
inquiry
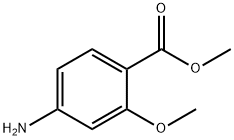
27492-84-8
317 suppliers
$6.00/5g

59338-85-1
13 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

59338-85-1
13 suppliers
inquiry