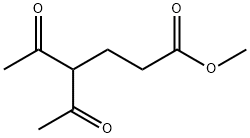
METHYL 4-ACETYL-5-OXOHEXANOATE synthesis
- Product Name:METHYL 4-ACETYL-5-OXOHEXANOATE
- CAS Number:13984-53-7
- Molecular formula:C9H14O4
- Molecular Weight:186.21
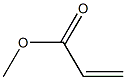
292638-85-8
3 suppliers
inquiry
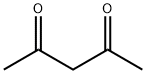
123-54-6
565 suppliers
$10.00/25ml
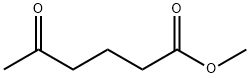
13984-50-4
59 suppliers
inquiry

13984-53-7
35 suppliers
$55.20/5g
Yield:-
Reaction Conditions:
Stage #1:acetylacetone with sodium in ethanol at 0; for 0.166667 h;
Stage #2:acrylic acid methyl ester in ethanol at 0 - 20; for 1.16667 h;Heating / reflux;
Stage #3: with acetic acid in ethanol
Steps:
1.a
Preparation 1; Synthesis of the Pyrrole of Formula (3): a) Preparation of the Compound of Formula (6): A 4.5 l Keller round-bottomed flask equipped with a reflux condenser, a thermometer, a dropping funnel and an argon conduit is loaded with ethanol (2 l). At ambient temperature, sodium (2.2 g) is gradually dissolved so as to give a clear solution which is cooled to 0° C. Acetyl acetonate (500.0 g, 5.0 mol) is added dropwise in 10 min, which results in gas being given off. 430 g of methyl acrylate are added dropwise to the light yellow solution at 0° C. in 10 min, which results in gas being given off. The reaction mixture is heated to ambient temperature and is then refluxed for 1 h. The conversion is followed by HPLC. The mixture is cooled to ambient temperature. Acetic acid (3 ml) is added and the ethanol is eliminated by distillation under reduced pressure. The distillation of the crude mixture (95-105° C., 2.5 mbar) gives a light yellow solution (747.8 g, 80%). The 1H NMR analysis shows that the crude product is an 1/1 mixture of compound (6) and of methyl 5-oxohexanoate.
References:
SANOFI PASTEUR SA US2008/242857, 2008, A1 Location in patent:Page/Page column 14-15

3395-91-3
266 suppliers
$5.00/5g

123-54-6
565 suppliers
$10.00/25ml

13984-53-7
35 suppliers
$55.20/5g

292638-85-8
3 suppliers
inquiry

123-54-6
565 suppliers
$10.00/25ml

13984-53-7
35 suppliers
$55.20/5g

292638-85-8
3 suppliers
inquiry

123-54-6
565 suppliers
$10.00/25ml

13984-53-7
35 suppliers
$55.20/5g
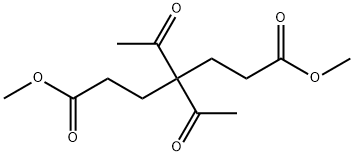
13984-59-3
1 suppliers
inquiry

15435-71-9
20 suppliers
$78.34/25 G
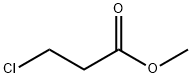
6001-87-2
77 suppliers
$45.00/25mg

13984-53-7
35 suppliers
$55.20/5g