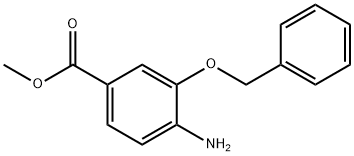
Methyl 4-amino-3-(benzyloxy)benzoate synthesis
- Product Name:Methyl 4-amino-3-(benzyloxy)benzoate
- CAS Number:475215-88-4
- Molecular formula:C15H15NO3
- Molecular Weight:257.28
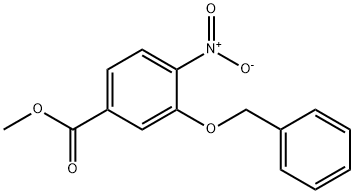
209528-69-8
15 suppliers
inquiry

475215-88-4
37 suppliers
$233.33/0.25g
Yield:475215-88-4 100%
Reaction Conditions:
with iron(0);glacial acetic acid at 20 - 40; for 4 h;
Steps:
4.11. Methyl 4-amino-3-benzyloxybenzoate (9b)
Iron powder (2.62 g, 46.9 mmol) was added carefully to a solution of methyl 3-benzyloxy-4-nitrobenzoate (20) (1.35 g, 4.69 mmol) in glacial acetic acid (20 mL). The resulting mixture was stirred for 2 h at room temperature and then for 2 h at 40 °C. The suspension was filtered with ethyl acetate over Hyflo Super Cel medium and the solvents were removed under reduced pressure. Water was added to the residue and the mixture was extracted four times with ethyl acetate. The combined organic layers were washed with brine, dried over magnesium sulfate and the solvents were removed under reduced pressure. Purification of the residue by flash chromatography on silica gel (petroleum ether/ethyl acetate 4:1) afforded methyl 4-amino-3-benzyloxybenzoate (9b) as colourless solid, yield: 1.21 g (100%), mp 99-100 °C (lit.:35 99-101 °C).UV (MeOH): λmax=230, 278 (sh), 306 nm. IR (ATR): ν=3475, 3447, 3353, 3199, 3080, 3024, 2985, 2945, 1681, 1618, 1578, 1522, 1499, 1438, 1399, 1363, 1315, 1298, 1264, 1219, 1186, 1148, 1106, 1016, 994, 927, 905, 889, 841, 824, 789, 765, 736, 692, 626 cm-1. 1H NMR (500 MHz, CDCl3): δ=3.86 (s, 3H), 4.20 (br s, 2H), 5.12 (s, 2H), 6.69 (dd, J=6.7, 2.0 Hz, 1H), 7.36 (tt, J=7.2, 2.5 Hz, 1H), 7.41 (tt, J=8.0, 2.2 Hz, 2H), 7.46 (d, J=7.4 Hz, 2H), 7.56 (m, 1H), 7.57 (dd, J=6.7, 1.8 Hz, 1H). 13C NMR and DEPT (125 MHz, CDCl3): δ=51.86 (CH3), 70.65 (CH2), 112.72 (CH), 113.41 (CH), 119.54 (C), 124.50 (CH), 127.97 (2 CH), 128.34 (CH), 128.75 (2 CH), 136.74 (C), 141.51 (C), 145.38 (C), 167.39 (CO). MS (EI): m/z (%)=257 (34, M+), 226 (6), 166 (44), 138 (9), 91 (100), 79 (6), 65 (9), 52 (5). Anal. Calcd (%) for C15H15NO3: C 70.02, H 5.88, N 5.44. Found: C 69.86, H 5.92, N 5.35.
References:
B?rger, Carsten;Kn?lker, Hans-Joachim [Tetrahedron,2012,vol. 68,# 33,p. 6727 - 6736] Location in patent:experimental part
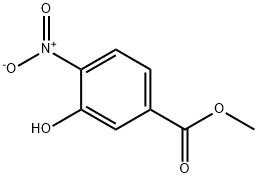
713-52-0
171 suppliers
$10.00/1g

475215-88-4
37 suppliers
$233.33/0.25g
![Benzoic acid, 4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-hydroxy-, methyl ester](/CAS/20210305/GIF/941715-62-4.gif)
941715-62-4
1 suppliers
inquiry

475215-88-4
37 suppliers
$233.33/0.25g
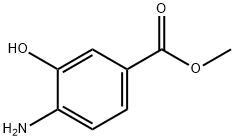
63435-16-5
168 suppliers
$8.00/1g

475215-88-4
37 suppliers
$233.33/0.25g
619-14-7
290 suppliers
$10.00/5g

475215-88-4
37 suppliers
$233.33/0.25g